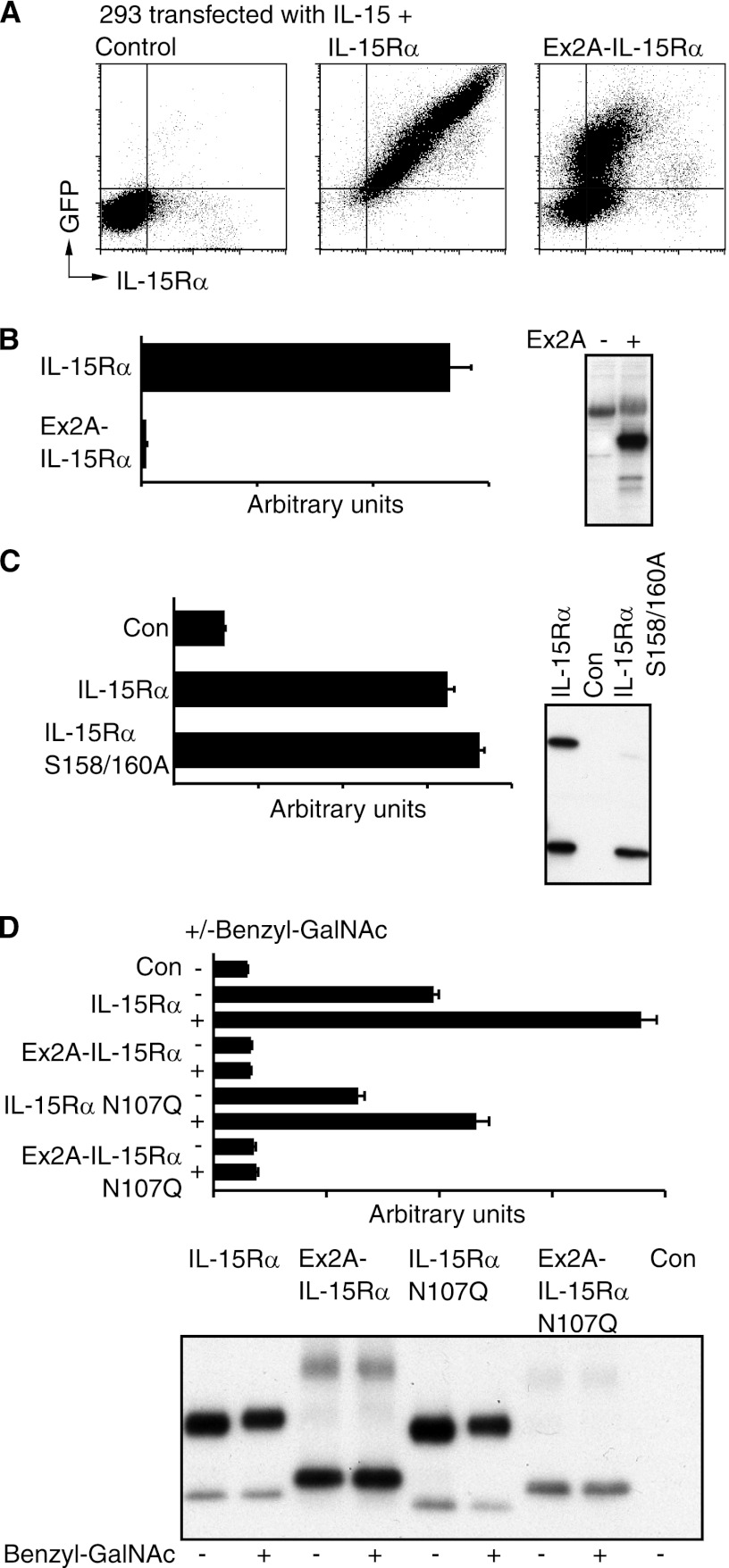
FIGURE 4.
Ex2A inhibits surface expression and prevents secretion of IL-15/IL-15Rα heterodimer. A, IL-15Rα with C-terminal GFP tags and IL-15 were co-expressed in 293 cells and stained for intracellular GFP and extracellular IL-15Rα. Increased GFP expression was accompanied by a smaller increase in the expression of extracellular IL-15Rα when Ex2A was present suggesting that Ex2A inhibited the surface export of IL-15/IL-15Rα heterodimer. B, no IL-15/IL-15Rα heterodimer was detected by ELISA in the supernatant when Ex2A was present. The panel on the right shows the levels of cell-associated IL-15Rα for this experiment. C, wildtype IL-15Rα without Ex2A as well as a construct with two amino acid substitutions (S158/160A) that lacked IL-15Rα modified by O-glycosylation similar to when Ex2A is present were coexpressed with IL-15 and analyzed by ELISA (graph) and Western blotting (panel). Secreted IL-15/IL-15Rα heterodimers were detected for both conditions suggesting that Ex2A-induced changes in the O-glycosylation of IL-15Rα and its secretion are not linked. D, both O- and N-glycosylation affect IL-15/IL-15Rα heterodimer secretion. The presence of the O-glycosylation inhibitor Benzyl-GalNAc increased levels of IL-15/IL-15Rα heterodimer secretion (graph) despite a lack of discernible effects on IL-15Rα migration on Western blots suggesting that an O-glycosylation of alternative proteins is involved in determining secretion levels. Expressions of an asparagine mutant (N107Q) that prevented N-glycosylation decreased IL-15/IL-15Rα heterodimer secretion suggesting a positive effect of IL-15Rα N-glycosylation on its secretion.