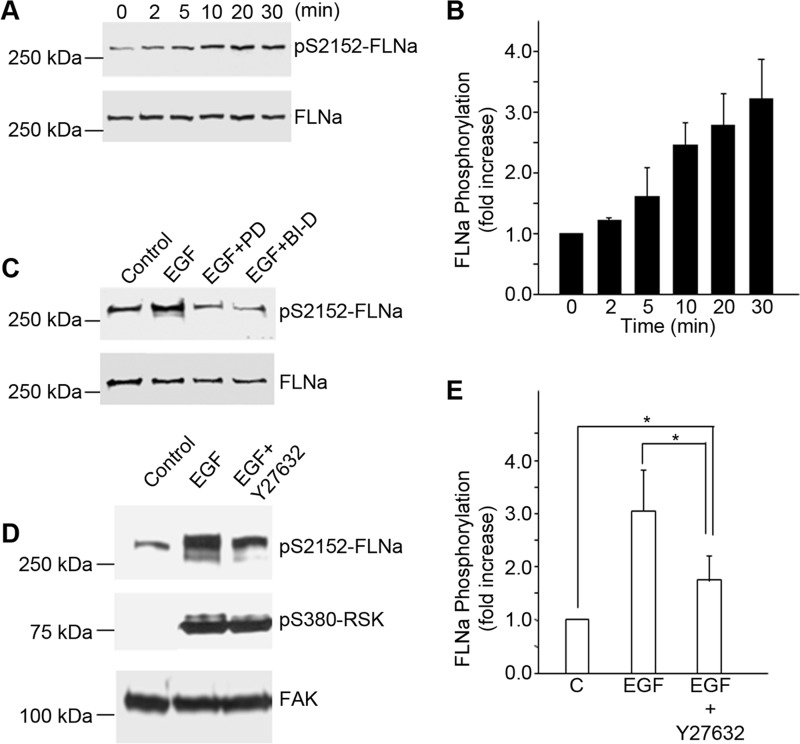
FIGURE 6.
EGF-mediated phosphorylation of FLNa is dependent on MEK/ERK/p90RSK and Rho/Rho kinase pathways. A, A431 cells were stimulated with EGF (3 nm) at the indicated times. Nuclear free cell lysates were immunoblotted using antibodies to phosphorylated FLNa. Staining for total FLNa served as a loading control. pS2152-FLNa, phospho-Ser-2152 FLNa. B, bands for phospho-FLNa were quantified and normalized to total FLNa. Values are means ± S.D. of three separate experiments. C, A431 cells were pretreated for 1 h with an inhibitor of MEK, PD184352 (PD; 2.5 μm), or with an inhibitor of p90RSK, BI-D1870 (BI-D; 10 μm), before stimulation with EGF (3 nm) for 20 min. Lysates were prepared and analyzed by Western blotting using antibodies to phosphorylated FLNa. Staining with antibodies to FLNa served as a loading control. This experiment is representative of three separate experiments. D and E, A431 cells were pretreated for 1 h with an inhibitor of Rho kinase, Y27632 (10 μm), before stimulation with EGF (3 nm) for 20 min. Lysates were prepared and analyzed by Western blotting (D) using antibodies to phosphorylated FLNa or phosphorylated p90RSK. pS380-p90RSK, phospho-Ser-380 p90RSK. Staining with antibodies to FAK served as a loading control (C). Bands for phosphorylated FLNA were quantified (E) and normalized to FAK. Values are means ± S.D. of four separate experiments. Statistical analysis to determine significance was performed using Student's t test. *, p < 0.05.