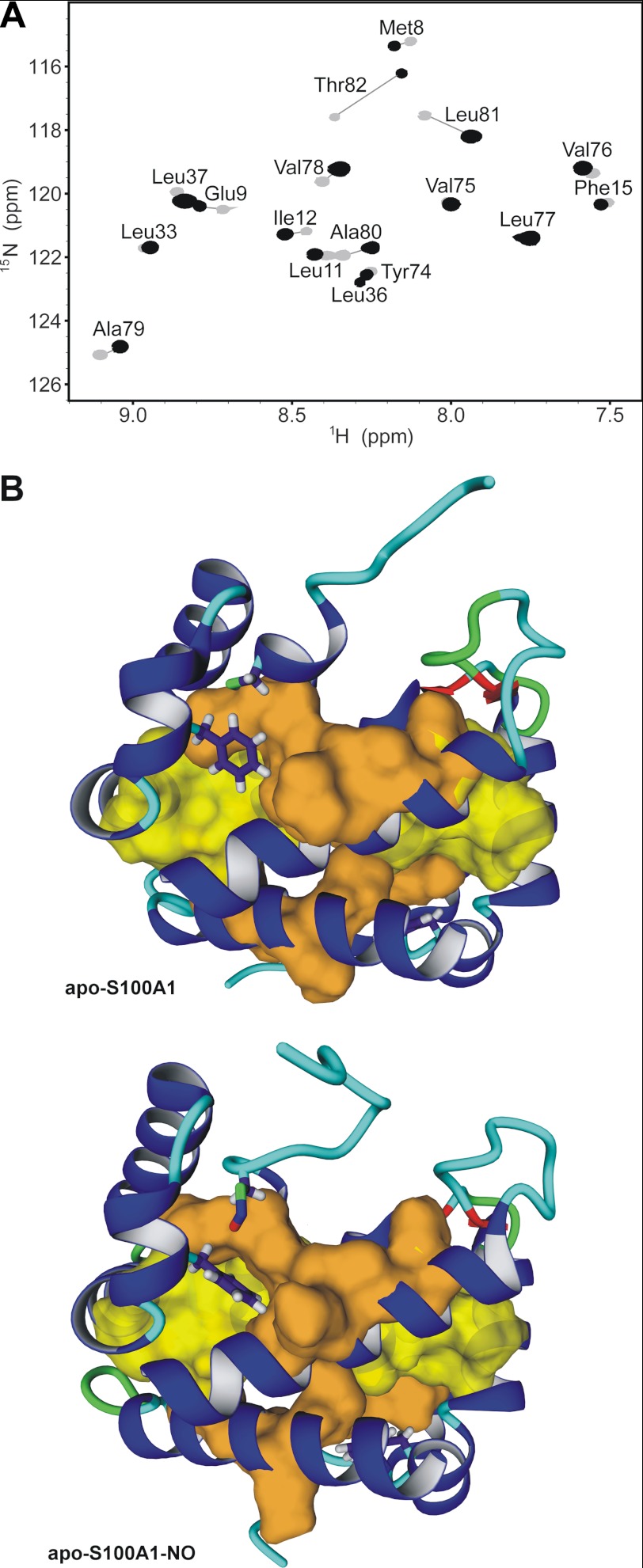
FIGURE 6.
Hydrophobic core packing in apo-S100A1 and apo-S100A1-NO proteins. A, overlay of two-dimensional 1H-15N HSQC NMR spectra acquired at 298 K on a Varian VNMRS 800 NMR spectrometer for apo-S100A1 (gray) and apo-S100A1-NO (black) after 6 months of storage at 4 °C in D2O solution. B, ribbon representation of the structures of apo-S100A1 (top) and apo-S100A1-NO (bottom). Side chains of residues Phe44, Cys85, and Cys85-NO are presented as sticks, and van der Waals radii for residues with highly protected amide protons (A) with negligible or large chemical shift perturbations are shown in yellow and orange, respectively.