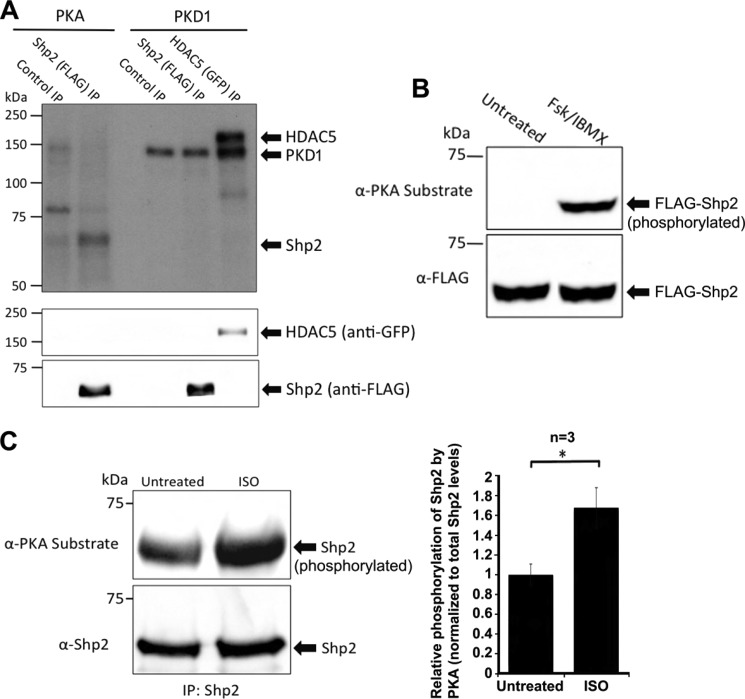
FIGURE 4.
Shp2 is a PKA, but not a PKD1 substrate. A, PKA, but not PKD1, phosphorylates Shp2 in vitro. Immunoprecipitated FLAG-tagged Shp2 from HEK293 cells was phosphorylated in vitro in kinase assay buffer supplemented with [γ-32P]ATP and bacterially purified recombinant PKA catalytic subunit (0.2 mg), or recombinant PKD1 (0.2 mg). Reactions were for 20 min at 30 °C and were terminated by washing twice with fresh kinase buffer prior to resuspension in Laemmli sample buffer. Incorporation of phosphate was determined by autoradiography following SDS-PAGE and transfer to nitrocellulose. GFP-HDAC5 (a well characterized PKD1 substrate) was used as a positive control in this experiment. Additionally, autophosphorylation of PKD1 is observed, indicating that PKD1 was active in this experiment. Immunoblotting confirmed similar levels of Shp2 in both the PKA and PKD1 phosphorylation reactions. A control FLAG-IP was also carried out using cell lysate where FLAG-Shp2 was not expressed. B, activation of PKA promotes Shp2 phosphorylation in HEK293 cells. Cells were treated with either DMSO (untreated) or forskolin (20 μm) and IBMX (75 μm) for 20 min to activate PKA. Western blotting using an anti-PKA-phosphosubstrate antibody was carried out following immunoprecipitation of FLAG-Shp2, SDS-PAGE, and transfer to nitrocellulose. The immunoblot was stripped and then probed for total levels of Shp2 with anti-FLAG antibody. C, activation of PKA promotes Shp2 phosphorylation in cardiac myocytes. Neonatal rat ventricular myocytes were treated with either DMSO (untreated) or isoproterenol (10 μm isoproterenol for 20 min) to activate PKA. Western blotting using an anti-PKA-phosphosubstrate antibody was carried out following immunoprecipitation of endogenous Shp2, SDS-PAGE, and transfer to nitrocellulose. The immunoblot was stripped and then probed for total levels of Shp2 with anti-Shp2 antibody. The relative increase in Shp2 phosphorylation by PKA in response to isoproterenol was quantified over three independent experiments. Error bars, indicate S.E. A p value of 0.05 is considered significant (*).