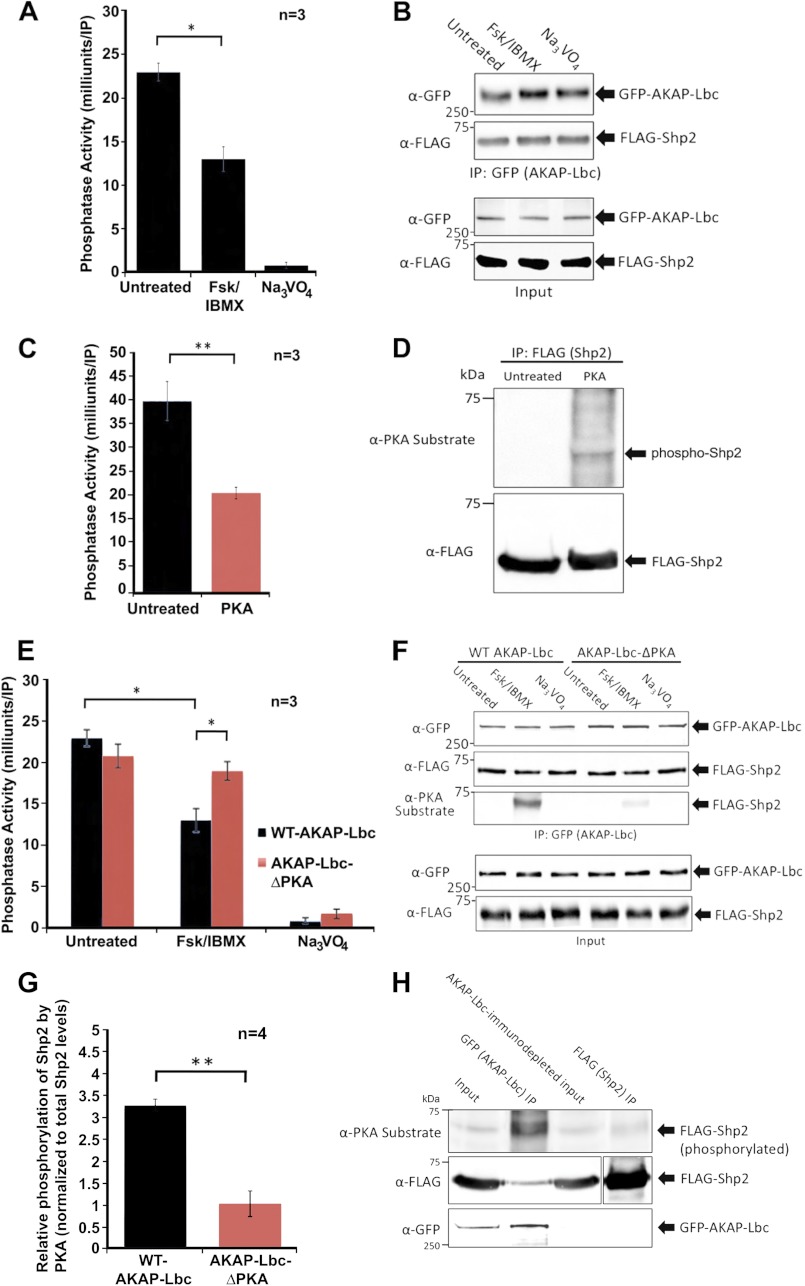
FIGURE 5.
Shp2 activity in the AKAP-Lbc complex is inhibited by PKA. A, PKA activation promotes a decrease in Shp2 activity in the AKAP-Lbc complex. HEK293 cells transfected for expression of AKAP-Lbc and FLAG-Shp2 were treated for 20 min with either DMSO (untreated), or with forskolin (20 μm) and IBMX (75 μm) to activate PKA. Subsequent AKAP-Lbc immunoprecipitations were used for in vitro measurement of PTP activity. All assays were performed in triplicate for three independent experiments. Sodium orthovanadate was used in all assays to determine that PTP activity was being measured. B, Western blot loading controls demonstrate even expression of AKAP-Lbc and Shp2. To confirm even levels of AKAP-Lbc and co-immunoprecipitating Shp2 under all conditions, each IP was equally divided into four tubes; three tubes were used for PTP assay, and the fourth was used for SDS-PAGE and Western blotting, probed with anti-GFP antibodies for AKAP-Lbc detection and anti-FLAG antibodies for Shp2 detection. C, in vitro phosphorylation of Shp2 by PKA inhibits Shp2 activity. Immunoprecipitated Shp2 was phosphorylated in vitro in kinase assay buffer supplemented with bacterially purified recombinant PKA C-subunit (0.2 mg) for 20 min at 30 °C. Parallel control reactions were performed where no PKA was added. Reactions were terminated by washing twice with fresh kinase buffer prior to PTP activity assay. All assays were performed in triplicate for three independent experiments. D, Western blot loading controls confirm phosphorylation of Shp2 by PKA and that equal amounts of Shp2 were present in all assay conditions. E, AKAP-Lbc-anchored PKA promotes a decrease in Shp2 activity. HEK293 cells were transfected for the expression of wild-type (WT)-AKAP-Lbc and FLAG-Shp2 or AKAP-Lbc-ΔPKA (a mutant that cannot bind PKA) and FLAG-Shp2. Prior to lysis, cells were treated for 20 min with either DMSO (untreated), or with forskolin/IBMX to activate PKA. AKAP-Lbc was immunoprecipitated, and immune complexes were used for in vitro measurement of PTP activity. All assays were performed in triplicate for three independent experiments. Sodium orthovanadate was used in all assays to confirm that PTP activity was being measured. F, Western blot loading controls demonstrate comparable expression of AKAP-Lbc-WT, AKAP-Lbc-ΔPKA, and associated Shp2 for all assays; however, PKA phosphorylation of Shp2 (assessed using the anti-PKA-phosphosubstrate antibody) is reduced in cells expressing AKAP-Lbc-ΔPKA compared with AKAP-Lbc-WT. G, Shp2 phosphorylation by AKAP-Lbc-anchored PKA is quantified using ImageJ software. H, Shp2 not associated with AKAP-Lbc is minimally phosphorylated by PKA, in contrast to Shp2 in the AKAP-Lbc complex. Prior to lysis, HEK293 cells expressing GFP-AKAP-Lbc and FLAG-Shp2 were treated for 20 min with forskolin/IBMX to activate PKA. AKAP-Lbc was immunoprecipitated, and subsequent AKAP-Lbc-immunodepleted lysate was used for immunoprecipitation of Shp2. Immune complexes were subjected to SDS-PAGE and transfer to nitrocellulose. Immunoblotting was performed with anti-PKA-phosphosubstrate antibody to determine the extent of Shp2 phosphorylation. Membrane was stripped and re-probed with anti-FLAG and anti-GFP antibodies to confirm the levels of Shp2 and AKAP-Lbc in each experimental condition. A shorter exposure is shown for total FLAG-Shp2 in the FLAG (Shp2) IP, compared with the other lanes (indicated by separation of the two exposures), due to relative high levels of total FLAG-Shp2 immunoprecipitated. Error bars, indicate S.E. p = 0.05 is considered significant (*), and p = 0.01 is considered very significant (**).