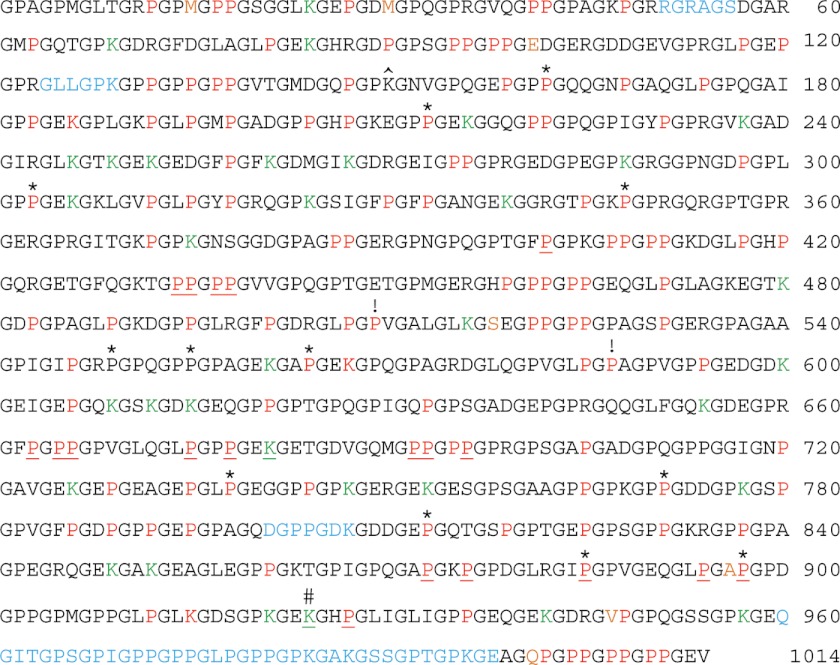
FIGURE 1.
Hydroxylated amino acid residues and saccharide attachments of the bovine placenta α1(V) collagen chain. Sequence coverage was 94%, with identification and mapping of 106 Y-position Hyp, 22 Gly-Hyp-Hyp X-position Hyp, 1 Gly-Hyp-Ala, 1 Gly-Hyp-Val, 3 Hyl, and 34 Glc-Gal-Hyl residues. Red, hydroxylated, non-glycosylated residues; green, Glc-Gal-Hyl residues; underlined, hydroxylated/glycosylated residues mapped in previous studies (4, 32). Sequences not identified in the course of MS analysis are in light blue. The seven COL1 amino acid residues that differ between human and bovine are orange. *, 12 Y-position Pro residues hydroxylated in human or bovine α1(V) COL1 domain but not the other. ^, Lys residue hydroxylated in human recombinant pro-α1(V) but not in bovine placenta α1(V). #, Hyl residues identified by Wu et al. (4) as being involved in interchain covalent cross-links. !, Hyp residues detected in the X-position of Gly-X-Y triplets lacking a Y-position Hyp. Amino acid residues are numbered from 1 to 1014, with residue number 1 being the first amino acid and residue number 1014 being the final amino acid residue of the COL1 triple helical domain.