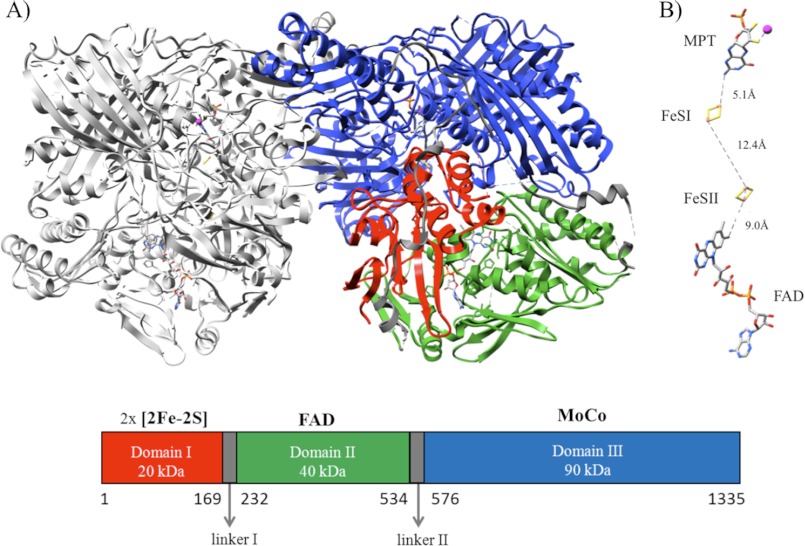
FIGURE 1.
A, shown is a ribbon representation of the mAOX3 crystal structure. The left monomer is in gray. The right monomer is shown with the three different domains colored as follows: domain I in red (residues Met-1–Pro-169), domain II in green (residues Thr-232–Leu-534), and domain III in blue (residues Leu-576–Val-1335). Domain III is separated from the FAD domain by a linker II region (Lys-535–Pro-575). The linker regions are represented in dark gray (linker I Ser-170–Asn-231). The two mouse AOX3 monomers are tightly bound, with the majority of contacts established by residues present in the domain containing the Moco binding site. The molybdenum atoms from the two monomers are more than 50 Å apart, and most likely, the two subunits work independently as shown previously for the R. capsulatus xanthine dehydrogenase (51). Homodimer approximate dimensions are 150 × 90 × 70 Å. B, arrangement and distances between the different protein cofactors are shown. MPT, the two distinct [2Fe-2S] centers and FAD.