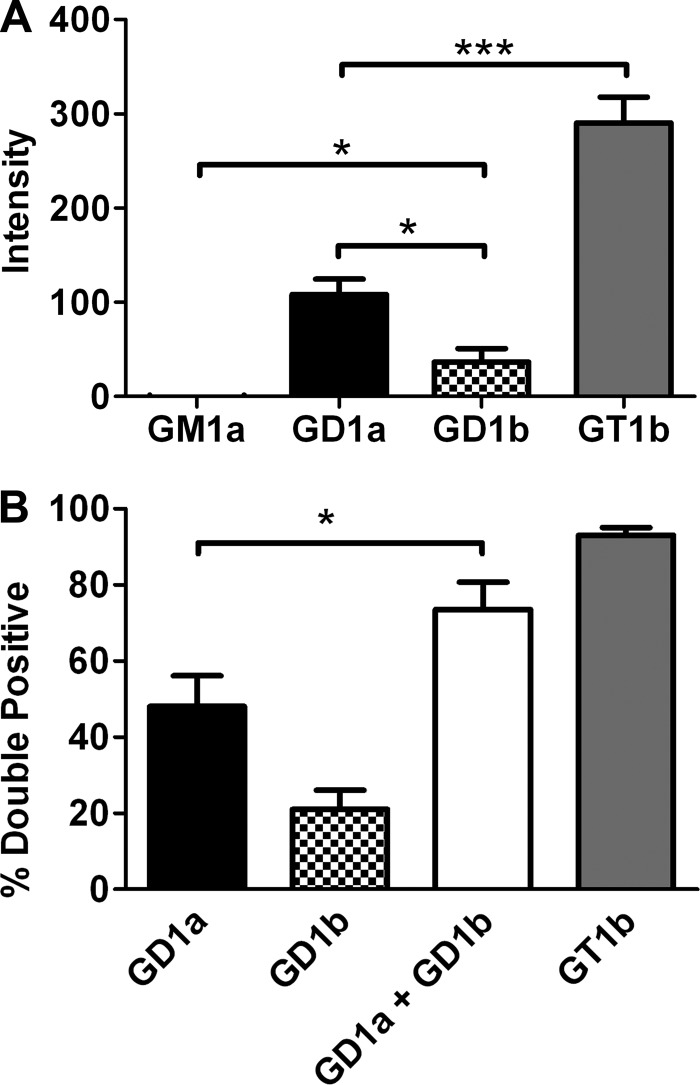
FIGURE 4.
HCR/C entry into N2A cells enriched with complex gangliosides. A, quantification of microscopic data. N2A cells were cultured with or without the indicated ganglioside (25 μg/well) for 4 h. 40 nm HCR/C was incubated with N2A cells with or without ganglioside for 30 min at 37 °C. Unbound HCR was washed away, and the cells were processed for microscopy. HCR/C was detected with an anti-HA antibody. Cells were stained for synaptophysin. Exposure times were constant between treatments. The average HCR/C fluorescence intensity was determined from three independent fields of consistent cellular morphology and density. The HCR/C intensity detected in N2A cells not supplemented with exogenous ganglioside was determined and subtracted from the ganglioside-dependent HCR intensity, and that value is presented here. Data presented are the average of three independent experiments. B, N2A cells were cultured with or without 5 μg of the indicated ganglioside for 4 h. 40 nm HCR/C was incubated with N2A cells with or without ganglioside for 30 min at 37 °C. Unbound HCR was washed away, and the cells were processed for microscopy. The percentage of cells in a given field that were double positive for HCR/C and synaptophysin was determined using ImageJ. Data were prepared using Excel and GraphPad Prism. The average of three independent experiments is shown. Asterisks denote statistical significance (*, p < 0.05; ***, p < 0.0001). A combination of GD1a and GD1b resulted in a statistically significant increase in the number of double positive cells relative to GD1a alone as determined by a two-tailed t test.