Abstract
Purification by Affinity Chromatography of the Glycine Receptor of Rat Spinal Cord (Pfeiffer, F., Graham, D., and Betz, H. (1982) J. Biol. Chem. 257, 9389–9393)
A γ-Aminobutyric Acid/Benzodiazepine Receptor Complex of Bovine Cerebral Cortex (Sigel, E., Stephenson, F. A., Mamalaki, C., and Barnard, E. A. (1983) J. Biol. Chem. 258, 6965–6971)
In 1982, two papers appeared in the Journal of Biological Chemistry that laid the foundation for our current understanding of certain molecular aspects of neurotransmission. Eric A. Barnard of the Imperial College of Science and Technology in the United Kingdom led a team to purify the first receptor for γ-aminobutyric acid (GABA; which later became known as the GABAA receptor) from bovine brain samples (1). In Germany, Heinrich Betz at the Max Planck Institute of Psychiatry and his team purified the glycine receptor from rat spinal cord (2). The biochemical purification of these proteins eventually led to the cloning of their genes; the analysis of the genes revealed the existence of the Cys loop ligand-gated ion channel superfamily of neurotransmitters.
By the early 1980s, the nicotinic acetylcholine receptor was the only neuronal ion channel that was understood in some detail, simply because it was plentiful in the organs of electric eels and fish and easy to purify. “There was very little other information and molecular detail about other neurotransmitter receptors,” explains F. Anne Stephenson at University College London, who was a member of the Barnard team. For this reason, researchers were hotly pursuing the purification of other neurotransmitter receptors.
Both the glycine and GABA receptors are ligand-gated ion channels that conduct chloride ions. When glycine and GABA bind to their respective receptors, the channels open up to allow chloride ions into the neuron. The ions cause the neuron to hyperpolarize and be less willing to undergo an action potential, so the glycine and GABA receptors are also known as inhibitory receptors.
The introduction of radioligand binding activity assays in the 1970s allowed researchers to measure the activity of neurotransmitter receptors in biochemical preparations. Researchers radiolabeled a molecule that bound to a receptor and tracked the radioactive signal to detect the receptor. The investigators on the two JBC papers here coupled the radioligand binding assay with affinity chromatography. Because a receptor naturally bound to its ligand, “you could use that property to purify the protein and study it in the test tube,” explains Richard Olsen, a molecular neuroscientist at the University of California at Los Angeles who was not affiliated with the two papers.
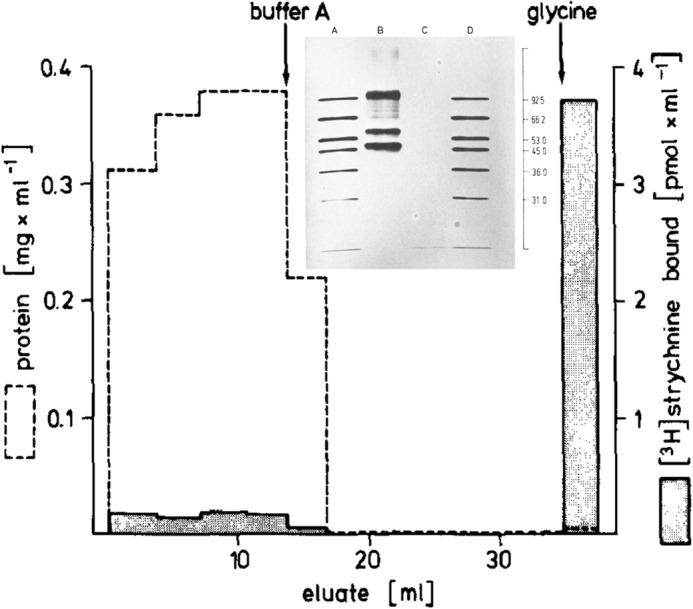
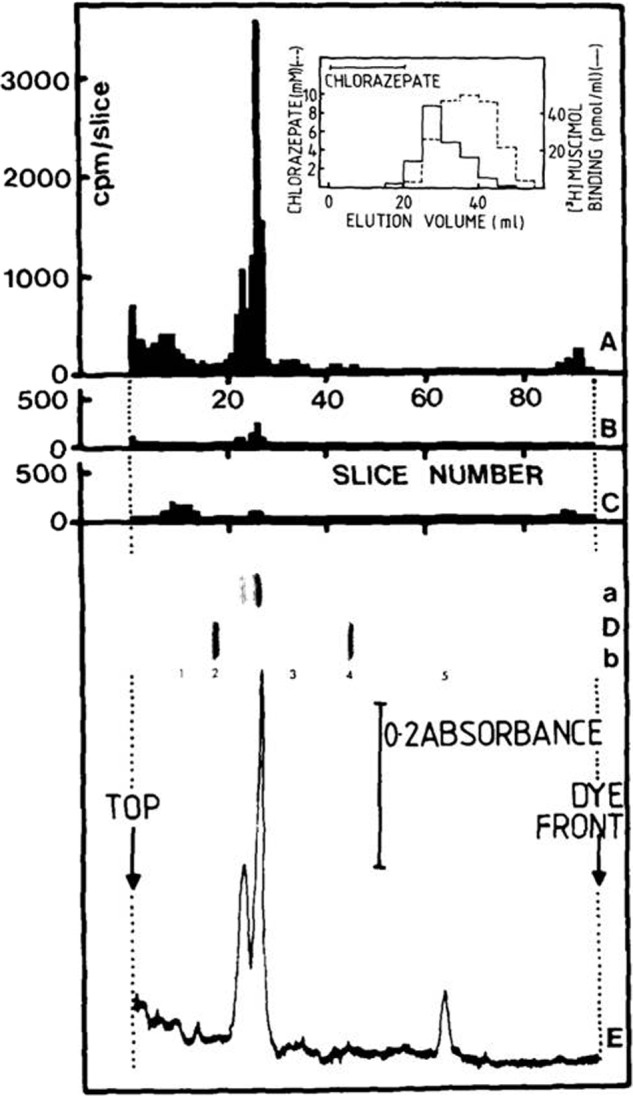
The Barnard and Betz teams knew it was going to be impossible to get the tiny molecules of radiolabeled GABA and glycine molecules on appropriate purification columns without affecting their structure and abilities to bind to the receptors, so they took another tack and used other molecules that bound to the receptors. For example, the poison strychnine binds to the glycine receptor with nanomolar affinity. The Betz team attached radiolabeled strychnine to beads (Fig. 1). They poured rat spinal cord preparations solubilized in detergent over the beads so that only the glycine receptor bound. After washing away all of the unbound material, the researchers eluted the purified receptor from the beads with glycine and cleaned up the preparation to get the pure protein. A similar approach was taken with the purification of the GABA receptor from bovine brain, for which the Barnard team used a benzodiazepine, a class of neuroactive molecules that include the famous Valium, on their purification column (Fig. 2). They used a radiolabeled benzodiazepine for detecting the GABA receptors after the purification process.
FIGURE 1.
The Barnard team used a radiolabeled benzodiazepine for detecting the GABA receptors after the purification process.
FIGURE 2.
The Betz team attached radiolabeled strychnine to beads to pull out the glycine receptor from rat spinal cord preparations.
The purified proteins revealed that the receptors were composed of multiple subunits, but as time and further research showed, all of the details in the two papers were not accurate. For the GABA receptor, Barnard's group reported two bands on SDS-polyacrylamide gels. These two polypeptides were used to obtain protein sequence, which then led to the cloning of the GABA receptor. However, molecular neuroscientists soon learned that the GABA receptor family actually consists of 19 different polypeptides classified into groups such as α, β, and γ. These polypeptides combine in different ways to form heteropentamers that result in various GABA receptor subtypes.
For the glycine receptor, Betz's group observed three polypeptides on a SDS-polyacrylamide gel at 48, 58, and 93 kDa, but research later showed that the 93-kDa band was a different protein called gephyrin. The protein is important because it “forms a matrix in the synaptic region to cluster receptor proteins together,” Olsen says. “Gephyrin was another breakthrough step in understanding neurotransmitter receptors that just happened to co-purify with the receptor [in the Betz study] because it binds the receptor tightly.”
The purification of the two receptors led to the cloning of their genes in 1987 (3, 4). Peter Seeburg, now at the Max Planck Institute for Medical Research in Germany, was enlisted by Barnard for the GABAA receptor gene cloning project. Seeburg explains that the cloning approach for the two receptors was similar. The proteins were broken into fragments by peptidases and cyanogen bromide. The small peptide fragments were sequenced. From the amino acid sequence, the researchers created complementary oligonucleotide probes. They then used the probes to screen cDNA libraries to find the gene that encoded the protein fragment.
Stephenson points out that the two JBC papers “had no functional data to show that they were actually ion channels,” but with the cloning of the genes, two avenues of research were now possible. First, the individual genes for the GABA and glycine receptors were coexpressed in Xenopus oocytes and analyzed by electrophysiology to prove the receptors were bona fide ion channels that conducted chloride ions. Second, the clones revealed the relationship between the ion channels.
Both glycine and GABA receptors are inhibitory chloride channels, so it was logical to expect them to be similar. However, when the researchers extended the sequence alignment to the nicotinic acetylcholine receptor, which conducts sodium ions, all three receptors had similar predictions in the number of membrane-spanning regions. Olsen says, “The amazing outcome was that the GABA and glycine receptors are related to each other in a family, and they are also in the same superfamily of genes as the nicotinic acetylcholine receptor.”
The three receptors bore similarities in their transmembrane domains but also had a conserved N-terminal motif called a Cys loop. For this reason, the receptor class is sometimes referred to as the Cys loop ligand-gated ion channel superfamily.
Both Olsen and Seeburg point out that these two papers and the subsequent cloning papers were the last of their kind. The two JBC papers represent “a superhuman effort to purify these proteins that took years,” says Olsen. “It's fair to say, after these were done and the protein sequences were used to identify the clones, almost nobody ever did that [approach] again. The technology had advanced so much that we didn't need to do protein purifications in order to clone.” Molecular biologists could now use homologies to pull out related sequences and exploit functional expression of cDNA and mRNA libraries.
Seeburg says that the GABA and glycine receptor purification and cloning stories reveal how resourceful nature is. “The interesting lesson, especially for the GABAA receptor, holds true for most of these ligand-gated ion channels. The lesson is that you have a single neurotransmitter like GABA but then you have a whole plethora of receptors that respond to the same neurotransmitter in different ways.”
Acknowledgments
F. Anne Stephenson at the University of London (JBC Associate Editor) nominated the papers as Classics, and Rajendrani Mukhopadhyay (ASBMB's Senior Science Writer) wrote the introduction.
REFERENCES
- 1. Sigel E., Stephenson F. A., Mamalaki C., Barnard E. A. (1983) A γ-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J. Biol. Chem. 258, 6965–6971 [PubMed] [Google Scholar]
- 2. Pfeiffer F., Graham D., Betz H. (1982) Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 [PubMed] [Google Scholar]
- 3. Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. (1987) Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature 328, 221–227 [DOI] [PubMed] [Google Scholar]
- 4. Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. (1987) The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328, 215–220 [DOI] [PubMed] [Google Scholar]


