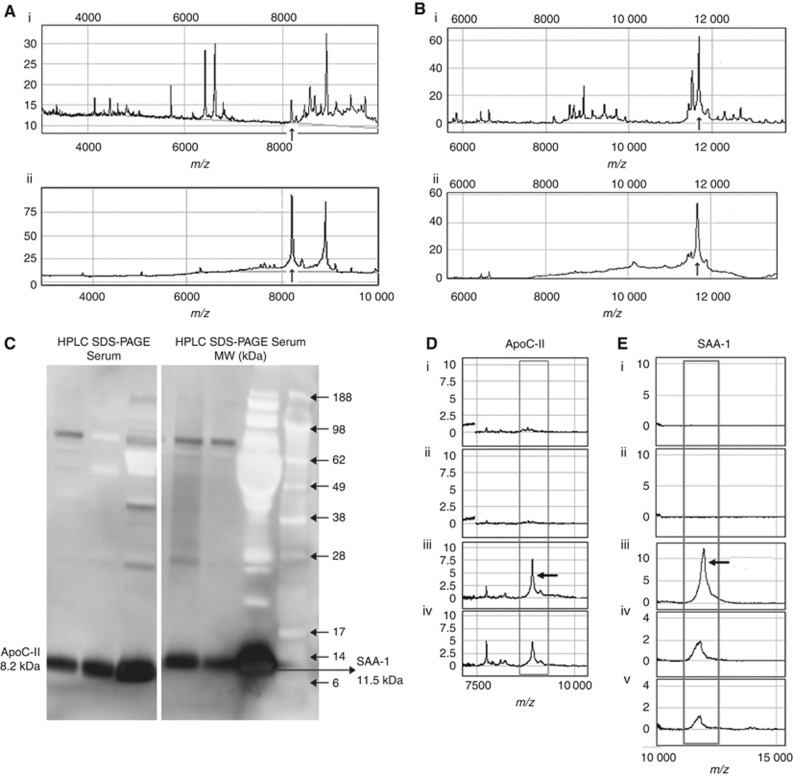
Figure 2.
Purification and identification of the proteins corresponding to m/z 8222 and 11 522 peaks. (Ai) Initial SELDI profile from serum; (Aii) semi-purified peak after HPLC fractionation showing the m/z 8222 peak (arrowed) selected for sequencing. (Bi) Initial SELDI for serum; (Bii) semi-purified sample for the m/z 11 522 peak (arrowed). (C) Western-blot lanes are labelled: serum (crude serum), HPLC (the HPLC fraction containing the peaks of interest), SDS–PAGE (the protein extracted from 1D-SDS–PAGE gel bands, which were subjected to protein sequencing and molecular weight (MW) (the protein standards, which are presented in kDa) for ApoC-II in the panel on the left and SAA-1 in the panel on the right. (D) Confirmation of m/z 8222 peak identity using SELDI immuno-adsorption on RS100 chips containing ApoC-II antibody. (Di) Affinity-purified IgG negative control; (Dii) unprocessed serum without antibody; (Diii) unprocessed serum with antibody; (Div) antibody with semi-purified ApoC-II from patient serum. (E) Confirmation of m/z 11 522 peak identity using SAA-1 antibody. (Ei) Affinity-purified IgG (negative control); (Eii) unprocessed serum without antibody; (Eiii) recombinant human SAA-1 (positive control); (Eiv) unprocessed serum with antibody; and (Ev) semi-purified SAA-1 from patient serum after HPLC purification with antibody.