Figure 7.
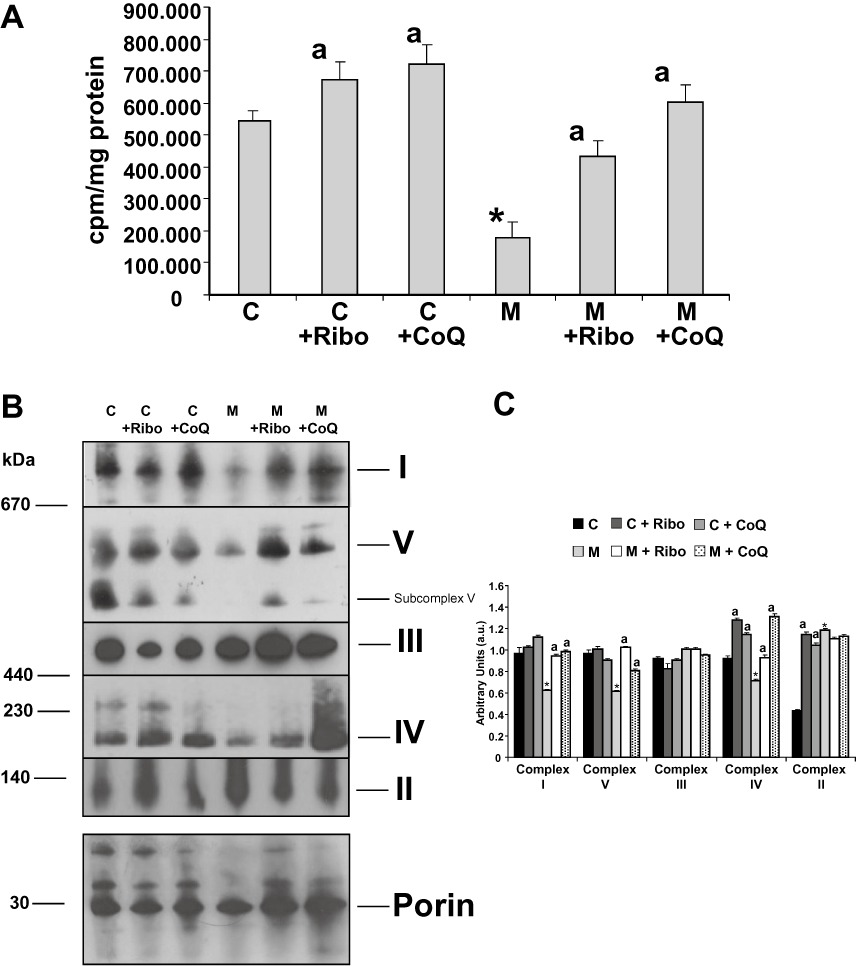
Mitochondrial protein synthesis and assembly of mitochondrial complexes. (A) Incorporation of [35S]-methionine into mitochondrial proteins. Control (C) and MELAS (M) fibroblasts supplemented with or without 0.06 µM riboflavin (Ribo) or 100 µM CoQ for 72 h were incubated with [35S]-methionine for 1 h in the presence of 100 µg mL−1 emetine or emetine plus 200 µg·mL−1 chloramphenicol. [35S]-methionine incorporation into mitochondrial proteins was measured by liquid scintillation counting. Results are expressed as cpm (cpm in the presence of emetine minus cpm in the presence of emetine plus chloramphenicol) mg-1of protein. Data represent the mean ± SD of three separate experiments. *P < 0.01 between control and MELAS fibroblasts. aP < 0.01, between the presence and the absence of riboflavin or CoQ. (B) Suppression of the defect in assembly of oxidative phosphorylation complexes in MELAS fibroblasts under riboflavin or COQ supplementation. Intact mitochondria from control (C) and MELAS (M) fibroblasts with or without 0.06 µM riboflavin (Ribo) or 100 µM CoQ supplementation for 72 h were analysed by BN-PAGE. The blots were incubated with antibodies against specific subunits of each of the five mitochondrial complexes (I–V). An antibody against porin, housekeeping mitochondrial protein, was used as loading control. ‘Subcomplex V’ indicates the presence of partially assembled complexes V in MELAS patients. (C) Densitometry of mitochondrial complexes signal normalized by porin was performed using ImageJ software. Data in arbitrary units (a.u.) represent the mean ± SD of two blots. *P < 0.01 between control and MELAS fibroblasts. aP < 0.01, between the presence and the absence of riboflavin or CoQ.
