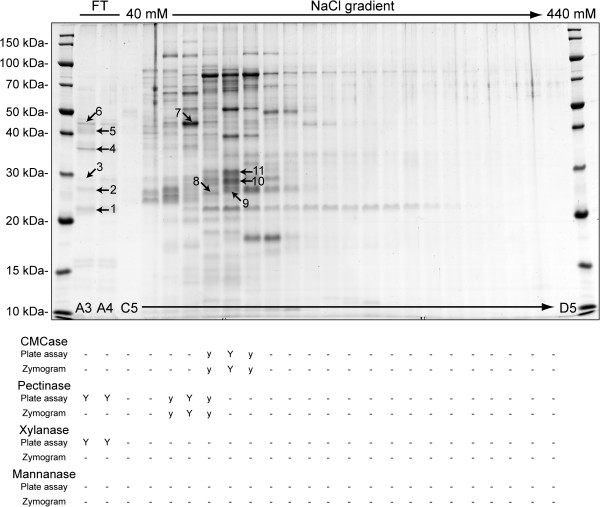
Figure 2.
Separation of proteins from P. cochleariae larval gut contents using a two-dimensional proteomics approach. Gut content proteins were separated by anion exchange chromatography (first dimension) and 12.5% SDS-PAGE (second dimension) followed by staining with colloidal Coomassie blue. Molecular weight markers in kilodaltons are indicated to the left of the gel. Proteins that bound to the anion exchange column were eluted between 40 and 440 mM NaCl. FT: Flowthrough. Fraction numbers from the anion exchange chromatography are indicated at the bottom of the gel (see chromatogram on Figure S1). Protein bands analyzed by mass spectrometry are designated by numbers. Enzymatic activities observed for each fraction by both diffusion assays and zymograms are indicated. ‘Y’ indicates strong activity and ‘y’ low activity.