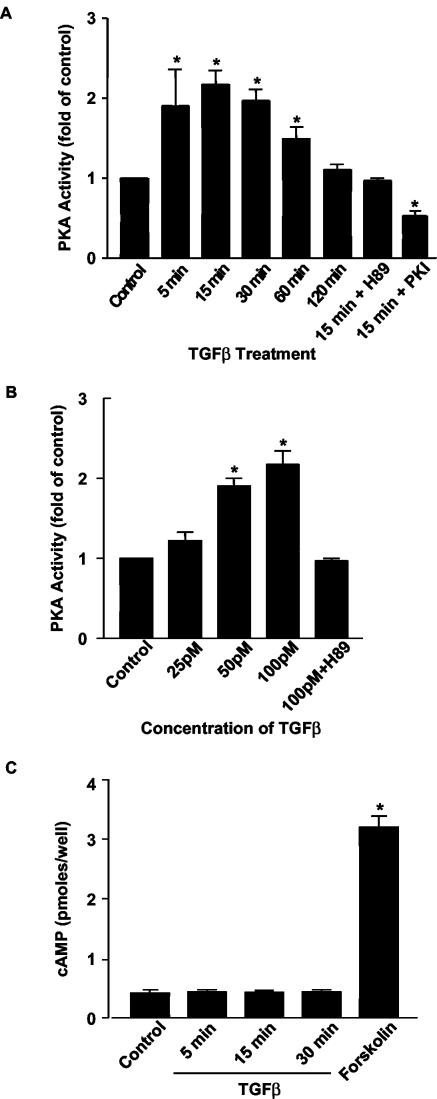
FIG. 1.
TGFβ activates PKA without increasing intracellular cAMP levels. (A and B) Mv1Lu cells were serum starved (24 h) and were then treated with TGFβ (100 pM) for the indicated time periods (A) or at the indicated doses for 15 min (B). In vitro kinase assays for PKA activity were performed with a biotinylated PKA peptide substrate (Kemptide [LRRASLG]; Promega). A specific PKA inhibitor H89 (3 μM) was used to pretreat some cells for 30 min. PKA activity was also measured in cells transfected with a pcDNA3.0 plasmid which expresses the specific PKA molecular inhibitor PKI. Results are expressed as increases over the control from three separate experiments (*, P < 0.05 versus the control). (C) TGFβ does not increase cAMP. In the presence of the phosphodiesterase inhibitor IBMX (100 μM), cAMP levels were measured in Mv1Lu cells after treatment with TGFβ (100 pM) or forskolin (10 μM) for 15 min by using a Biotrak enzyme immunoassay assay kit (Amersham). Results are from three separate experiments (*, P < 0.05 versus the control).