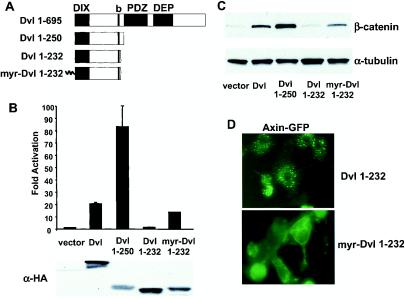
FIG. 1.
Identification of a hyperactive Dvl mutant that stimulates the β-catenin signaling pathway. (A) Schematic representations of mouse Dvl 1 and its mutants. Domains that are conserved among Dvl molecules in different species are highlighted. The basic region (b) is a region N-terminal to the PDZ domain that contains multiple basic amino acid residues. (B) Effects of Dvl mutants on LEF-1-mediated transcription. 293 cells were plated in 24-well plates and transfected with 0.01 μg of LEF-1 expression plasmid, 0.01 μg of cytomegalovirus-Renilla luciferase expression plasmid, 0.1 μg of LEF-1 luciferase reporter plasmid, and 0.1 μg of Dvl expression plasmid. Empty vector DNA was added to equalize the total amount of DNA (0.5 μg/transfection). The luciferase activities (top panel) were normalized to the Renilla luciferase activities. Each experiment was carried out in triplicate, and error bars represent standard deviations. Dvl proteins were tagged with the HA epitope at their carboxyl termini, and protein expression was examined by immunoblot analysis with anti-HA antibodies (bottom panel). Note that expression of Dvl 1-250 was significantly lower than that of wild-type Dvl. (C) Effects of Dvl mutants on β-catenin stabilization. 293 cells were transfected with the indicated plasmids. Forty-eight hours after transfection, cells were subjected to subcellular fractionation. Equal amounts of cytosolic extracts were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-β-catenin antibodies (top panel). α-Tubulin levels were determined by immunoblotting with anti-α-tubulin antibodies as an internal loading control (bottom panel). (D) Recruitment of Axin-GFP to the plasma membrane by myristoylated Dvl 1-232. Axin-GFP and Dvl 1-232 or myristoylated Dvl 1-232 were coexpressed. The subcellular distributions of Axin-GFP were examined by fluorescence microscopy.