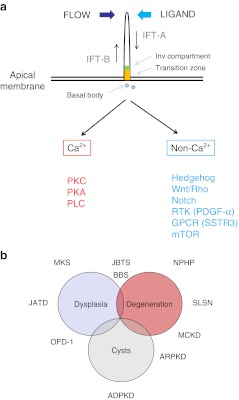
Fig. 1.
a Cilia structure and signaling. Primary cilia extend as apical structures with a microtubular core in polarized kidney epithelial cells. They are assembled, disassembled and maintained by a process called intraflagellar transport (IFT). Complex B proteins undergo anterograde transport and complex A undergo retrograde transport. The transition zone (orange) and inversin (Inv, green) ciliary sub-compartments are shown. Cilia signaling can be activated by mechanical bending, flow or ligand binding to a variety of membrane receptors expressed in the ciliary membrane. Cilia signaling can be usefully divided into Ca2+ or non- Ca2+ mediated pathways. Apart from stimulating Ca2+ release from intracellular stores, Ca2+ increases could activate protein kinase C (PKC), protein kinase A (PKA) and phospholipase C (PLC) signaling. Non- Ca2+ signaling could involve multiple receptors including developmental pathways such as Hedgehog, Wnt and Notch, as well as those regulating cell division or cell size such as PDGF-α (platelet-derived growth factor- alpha), SSTR3 (somatostatin receptor 3) or mTOR (mammalian target of rapamycin). b Disease spectrum of the ciliopathies. The phenotypic spectrum of the ciliopathies may overlap and ranges from organ dysplasia, degeneration or fibrosis to cystic change. A number of the common ciliopathies and their typical phenotypes are shown on the diagram