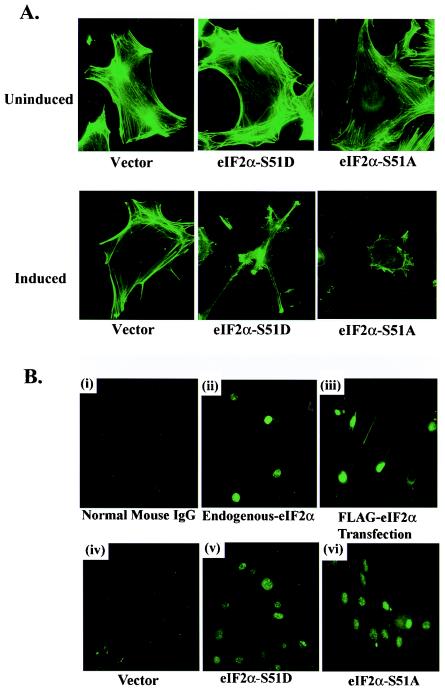
FIG. 2.
(A) Polymerized F-actin filament organization in FLAG eIF2α variant-expressing cells. Vector-, FLAG S51A-, and FLAG S51D-expressing cell lines grown on glass coverslips were permeabilized and stained with fluorescein isothiocyanate-conjugated phalloidin proteins that specifically bind to polymerized F-actin filaments on day 0 or day 6 postinduction. Actin filaments were visualized by immunofluorescence microscopy. (B) Subcellular localization of endogenous and FLAG-tagged eIF2α. Staining of 3T3 L1 cells with nonspecific normal mouse immunoglobulin G was included as a negative control (i). Expression and localization of endogenous eIF2α in normal 3T3 L1 cells were determined using immunofluorescence with a MAb directed against native eIF2α (ii). Localization of FLAG-tagged eIF2α in normal 3T3 L1 cells was determined by immunofluorescence with a MAb directed against the FLAG epitope (clone M5; Sigma) following transient transfection for 48 h with a vector expressing FLAG-tagged WT eIF2α (iii). Localization of inducible FLAG-tagged eIF2α variants in empty vector-, FLAG eIF2α-S51A-, or FLAG eIF2α-S51D-expressing 3T3 L1 cell lines was determined by immunofluorescence with an antibody to the FLAG epitope following 6 days of induction in the absence of DOX (iv to vi). IgG, immunoglobulin G.