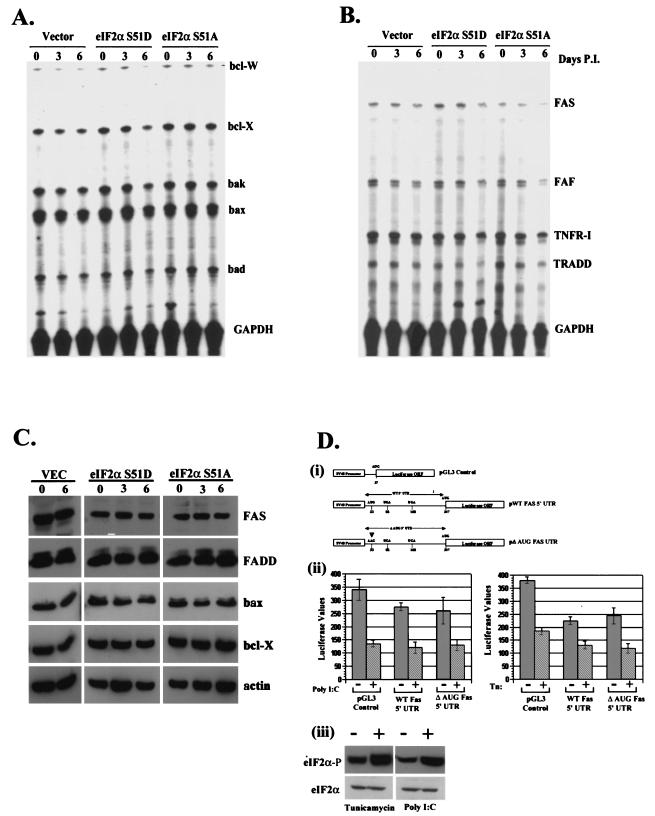
FIG. 5.
(A) Phosphorylation of eIF2α does not regulate the transcription of key apoptotic genes. Total RNA was harvested from vector-, S51A-, and S51D-expressing cell lines after 0, 3, or 6 days of induction and quantified by an RNase protection assay with radiolabeled probes derived from the indicated genes. (B) Total RNA was harvested from cells as described for panel A and quantitated by an RNase protection assay with radiolabeled probes derived from the indicated cytoplasmic apoptotic signaling genes. (C) eIF2α phosphorylation does not affect the level ofapoptotic proteins. Total-cell lysates harvested from vector-, S51A-, or S51D-expressing inducible cell lines on 0, 3, or 6 days postinduction were resolved by SDS-PAGE and probed with antibodies to the indicated proteins. (D) Translational regulation of Fas requires elements in addition to the 5′ UTR. Pooled populations of normal 3T3 L1 cells stably expressing luciferase, luciferase fused in frame with the WT Fas 5′ UTR, or luciferase fused to a mutant Fas 5′ UTR (i) were transfected with either 10 μg of poly(IC) or 10 μg of tunicamycin/ml to induce phosphorylation of eIF2α. Relative luciferase counts were determined after 3 h in the presence or absence of treatment (ii). Additionally, whole-cell lysates derived from normal 3T3 L1 cells or cells treated with tunicamycin or poly(IC) were resolved by SDS-PAGE and probed with antibodies recognizing total or phosphorylated eIF2α (iii). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; P.I., postinduction; SV40, simian virus 40.