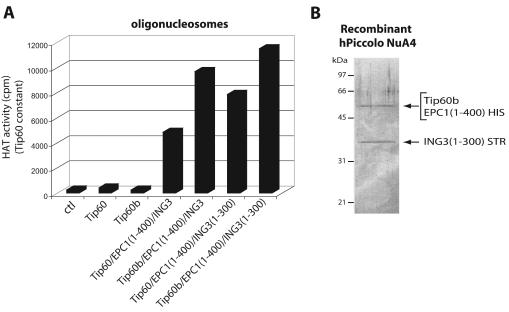
FIG. 6.
Conservation of the core nucleosomal acetyltransferase trimeric complex, Piccolo NuA4, from yeast to humans. (A) Coexpression of EPC1 and ING3 with His-tagged Tip60 and Tip60b in bacteria enable their HAT activity on chromatin substrates. Tip60(b) were affinity purified from bacteria expressing each protein alone or in conjunction with EPC1 conserved N-terminal domain [EPC1(1-400)] and ING3 with or without its C-terminal PHD finger [ING3(1-300)]. Relative nucleosomal HAT activity of affinity-purified HIS-tagged Tip60 and Tip60b were measured by liquid scintillation counting of HAT assays on oligonucleosomes while the amount of Tip60(b) was kept constant (evaluated by Western blot). Note that Tip60 and Tip60b are not able to acetylate chromatin by themselves and that ING3 PHD finger is not required for nucleosomal HAT activity. Average counts per minute (cpm) from four different HAT assays are shown (ctl, 276 ± 74; Tip60, 425 ± 162; Tip60b, 273 ± 25; Tip60/EPC1(1-400)/ING3, 4,872 ± 271; Tip60b/EPC1(1-400)/ING3, 9,714 ± 531; Tip60/EPC1(1-400)/ING3(1-300), 7,876 ± 873; Tip60b/EPC1(1-400)/ING3(1-300), 11,522 ± 1,417). (B) Tip60, EPC1 and ING3 form a stable trimeric complex when coexpressed in bacteria. Protein extract from bacteria coexpressing EPC1(1-400) with a C-terminal His6 tag, ING3 (1-300) with a C-terminal Strep-II tag and untagged full-length Tip60b was successively affinity purified on Talon cobalt and Strep-Tactin resins and analyzed on gel after Coomassie blue staining. The presence of all three proteins was confirmed by Western blot and HAT activity (not shown).