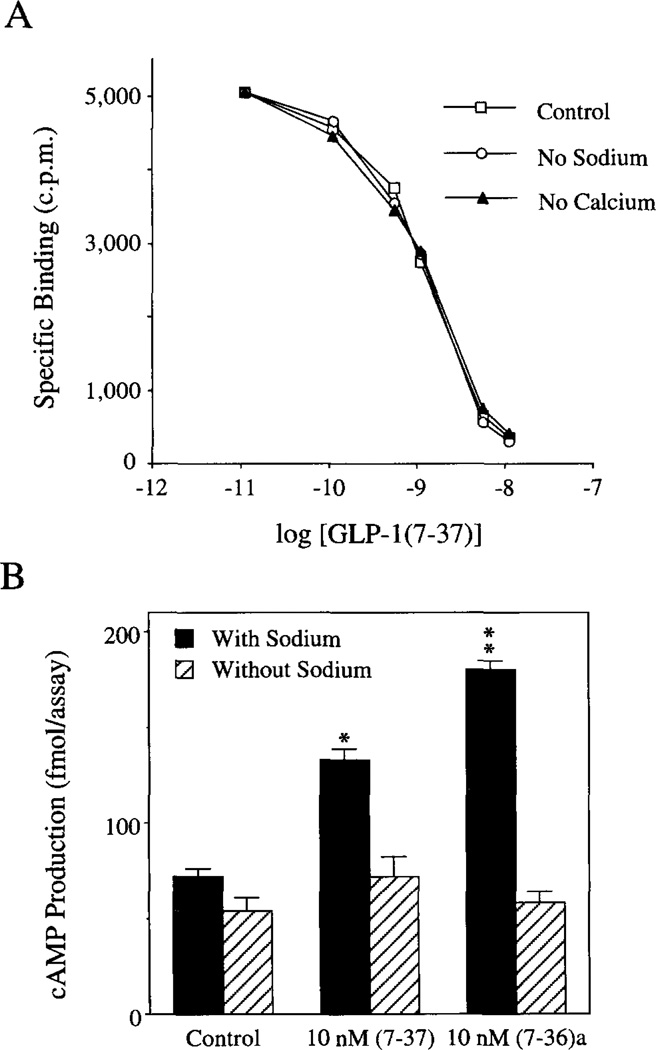
Fig. 2. Effects of ion substitution on GLP-1 binding and cAMP production.
Panel A, omission of Na+ or Ca2+ from the extracellular solution had no effect on the binding of GLP-1 to its receptor. COS-7 cells were transiently transfected with the rat islet GLP-1 receptor cDNA, and the binding of 125I-GLP-1(7-37) was determined 3 days post-transfection. Illustrated are displacement curves generated under conditions in which the binding of l25I-GLP-1(7-37) was competed for by prior equilibration of cells with the indicated concentration of non-radioactive GLP-1(7-37). Under control conditions in which the extra-cellular solution contained 138 mm Na+ and 2.6 mm Ca2+, approximately 20% of added tracer was bound specifically, and the IC50 for displacement of radioligand by nonradioactive peptide was determined to be approximately 2 nm. Neither Na+ omission (by replacement of Na+ with NMG) nor Ca2+ omission (by replacement of Ca2+ with Mg2+) influenced either the total binding activity or the IC50 value for displacement of radioligand. Nearly identical results were obtained using 125I-GLP-1(7-36)amide as radioligand and nonradioactive GLP-1(7-36)amide as displacer (data not shown). Panel B, the stimulation of βTC6 cell cAMP production by GLP-1 was dependent on extracellular Na+. Cells were incubated without (Control) or with GLP-1 for 30 min at 23 °C in extracellular solution containing 0.8 mm glucose, 100 µm IBMX, 2.6 mm CaCl2, and either 138 mm NaCl or 138 mm NMG (no Na+). Total cAMP production was determined by radioimmunoassay. Statistical significance relative to the control was evaluated by the t test (*,p ≤ 0.05; **,p ≤ 0.01).