Table 2.
Scope of the γ-Quinonylation Reaction.a
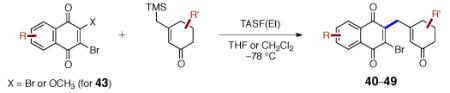
| entry | product and yield | entry | product and yield |
|---|---|---|---|
| 1 |
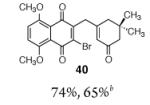
|
2 |
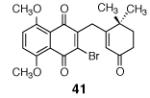
|
| 3 |
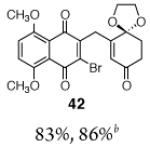
|
4 |
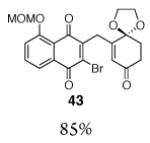
|
| 5 |
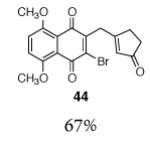
|
6 |
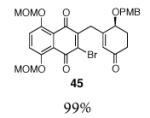
|
| 7 |
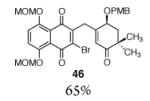
|
8 |
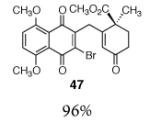
|
| 9 |
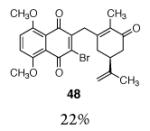
|
10 |
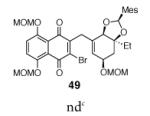
|
conditions: TASF(Et) (1.1 equiv), CH2Cl2, –78 °C.
TBAT (1.1–1.5 equiv), CH2Cl2, 0 °C.
none detected by 1H NMR analysis of the unpurified reaction mixture.
