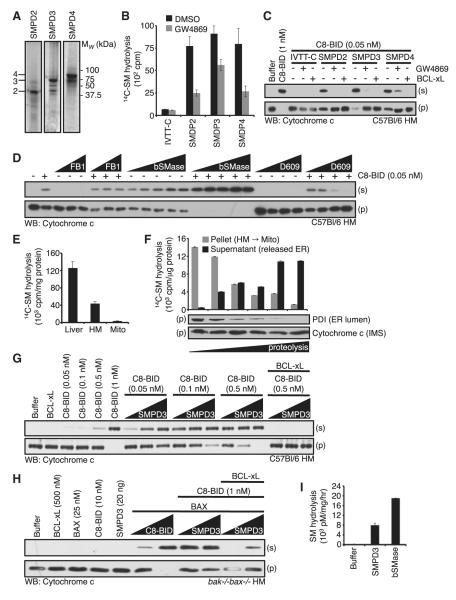
Figure 2. A Neutral SMase Activity Cooperates within the BCL-2 Family to Promote MOMP.
(A) SMPD2-4 IVTT with 35S-Met were subjected to SDS-PAGE. Lines denoting 2, 3, and 4 indicate full-length products.
(B) Nonradioactive IVTT reactions were performed and subjected to 14C-SM hydrolysis ± 10 μM GW4869.
(C) Nonradioactive IVTT reactions were performed and combined with WT HM fractions. C8-BID (1 nM for control; 0.05 nM for IVTT combinations); GW4869 (25 μM) and BCL-xL (50 nM).
(D) WT HM fractions were pretreated with FB1 (1, 5, 10, and 25 μM), bSMase (0.0001, 0.0005, 0.0010, and 0.0025U) or D609 (1, 5, and 25 μM) for 1 hr at 37°C before the addition of 0.05 nM C8-BID.
(E) Liver homogenate, solubilized HM fraction, or solubilized mitochondria (1 μg) were subjected to 14C-SM hydrolysis.
(F) HM fraction was subjected to limited proteolysis (0, 1, 2, 5, 10, and 20 min) on ice, and fractionated into pellet (mitochondria) and supernatant (contains released μS) before 14C-SM hydrolysis and western blot.
(G) WT HM fractions were combined with C8-BID (0.05, 0.1, 0.5, and 1 nM) or as indicated in the presence of SMPD3 (1, 5, and 20 ng) and BCL-xL (500 nM).
(H) bak-/-bax-/- HM fractions treated with SMPD3 (20 ng; or 1, 20 ng), BAX (25 nM), C8-BID (10 nM; or 1, 10 nM; or as indicated), and BCL-xL (500 nM).
(I) SMPD3 was tested for 14C-SM hydrolysis and compared to bSMase. All data are reported as ± SD.
See also Figure S2.