Abstract
Objective
Evaluate potential mechanisms promoting AAA development with tobacco smoke (TS) exposure.
Methods and Results
Experiments used the elastase perfusion (EP) model of AAA with smoke-free controls. The effect of TS-exposure was evaluated in C57/Bl6 mice, after broad-spectrum matrix metalloproteinase (MMP)-inhibition with doxycycline and in mice deficient in MMP-9, MMP-12, Cathepsin-S and Neutrophil Elastase. Preparations of washed marrow, spleen and peripheral blood leukocytes were transferred to smoke-free mice from 6 week TS-exposed mice or smoke-free mice. All mice were sacrificed 14 days after EP and the percent change in aortic diameter (%ΔAD) calculated. Before EP, there were no ultrastructural changes, by electron microscopy, in the aorta after TS-exposure.
Neither doxycycline nor any specific elastase deficiency was effective at preventing an increased %ΔAD in TS-exposed animals. Smoke-exposure for 6 weeks increased the %ΔAD after a smoke-free interval of up to 6 weeks before EP. Leukocyte preparations from TS-exposed mice localized to AAA and increased the %ΔAD in smoke-free mice.
Conclusions
The effect of TS on the development of AAA is not dependent on the activity of elastolytic enzymes, and persists for long periods despite cessation of TS. Alterations in leukocyte response to aortic injury appear to mediate this effect.
Keywords: Aneurysms, Immunologic techniques, Leukocytes, Metalloproteinases, Smoking
Active cigarette smoking is well recognized to enhance many vascular diseases including atherosclerosis and intimal hyperplasia, although not to the extent that it increases the risk of abdominal aortic aneurysms (AAA).1 In addition, the acceleration of arterial occlusive disease processes by tobacco smoke (TS) is thought to substantially abate shortly after quitting smoking.2 On the other hand, the increased risk of development of an AAA following TS exposure appears to persist for decades even in the absence of ongoing smoking.
Based on prolonged periods of smoke exposure in mice during studies of smoke-induced pulmonary disease, it has been recognized that smoke exposure alone did not appear to appreciably alter aortic morphology in wild type mice.3 However, in a modified mouse model of AAA, smoke exposure started shortly before elastase perfusion (EP), and continued throughout AAA development caused a much larger aneurysm to develop than elastase perfusion alone.4 These AAAs were associated with increased matrix damage, particularly increased elastic fiber degradation, and these findings have been confirmed in other AAA models.5 Others have shown in mice that antenatal exposure to TS can alter vascular physiology in the adult mouse in the absence of continued smoke exposure.6
Mechanisms of aneurysmal degeneration have focused on the inflammation found prominently in the media of the AAA and matrix proteases, particularly metalloproteases (MMP) with elastolytic activity. There have been divergent findings regarding the enhancement of MMP activity in the aortas of smoke-exposed animals.4, 5 In this study, we looked to evaluate potential mechanisms of smoke related AAA enhancement and determine whether the changes effected by smoke exposure are durable in the model.
Methods
Experimental Animals
Five strains of mice were used in these experiments, including wild-type 129/SvEv (Taconic, Hudson, NY) and C57/Bl6 (Jackson Labs, Bar Harbor, ME). MMP-9 -/- mice were bred at Washington University on both the 129/SvEv and the C57Bl/6 backgrounds, and MMP-12 -/-, neutrophil elastase -/- and Cathepsin-S -/- mice were bred at Washington University on the C57/Bl6 background. Throughout the experimental studies, all mice were allowed free access to food and water. Animals treated with doxycycline received a calculated dose of 30mg/kg/day in their drinking water as previously described.7 All experiments and animal care were conducted under protocols approved by the Washington University Animal Studies Committee.
Elastase-Induced AAAs
Adult male mice were subjected to transient perfusion of the abdominal aorta with elastase to induce AAAs, as previously described.4 Briefly, after sedation with pentobarbital (1.5mg/25g intraperitoneal), the infrarenal aorta was exposed through a midline laparotomy and the external aortic diameter (AD) was measured under physiologic blood pressure. The infrarenal aorta was temporarily isolated and perfused with a saline solution containing 0.07 units/ml of type I porcine pancreatic elastase (PPE; E-1250, Sigma, St. Louis, MO). All experiments were performed with a single PPE preparation derived from the same commercial source and lot. Following aortic perfusion, the laparotomy was closed, and the animal returned to standard housing. Mice were thereafter maintained in smoke-free conditions for an additional 2 weeks.
Smoke Exposure
C57Bl/6 mice were subjected to TS exposure according to a standard cigarette smoking protocol, as previously described.4 Using specially designed cages, animals were exposed to the smoke from 3 University of Kentucky 2R4F research cigarettes (filter removed) for one hour per day for 6 days per week. Littermate mice exposed to smoke-free conditions with filtered air and maintained with identical diets during the same period of time served as controls for TS-exposed mice. During the course of these experiments, we observed no differences in peri-procedural technical complications, tolerance for anesthesia, or immediate mortality between smoke-exposed and smoke-free mice.
Final Aortic Diameter Measurement and Specimen Collection
Two weeks following elastase perfusion, mice were again anesthetized with intraperitoneal pentobarbital, the abdomen was reopened, and the final AD was measured. Animals were then humanely sacrificed with intravenous pentobarbital (25mg) and the entire infrarenal aorta was harvested for RNA extraction, protein extraction or histology.
Measurement of Urinary Cotinine
Urine was collected from the mice at perfusion while under anesthesia, or at harvest. Urine was stored sterile until assayed. The Salimetrics (State College, PA) competitive enzyme immunosorbant assay was used to measure the concentration of cotinine in these specimens. Diluent for all specimens and controls was substituted with Surine Urine Matrix solution (Dyna-Tek). Samples from mice actively exposed to TS were assayed at a dilution of 1:4; all other specimens were assayed neat.
Protein Assays
Aortas from mice exposed to tobacco smoke for 6 weeks were harvested and the tissue was homogenized in 200μl RIPA buffer with protease inhibitor cocktail, and incubated on ice for 45min. The tubes were centrifuged at 10000rpm for 30min at 4°C, and the supernatant collected.
To determine the concentration of thiobarbituric acid reducing substances, 25μl of the protein extract was assayed with thiobarbituric acid at 100° C for 1 hour (Cayman Chemicals), then absorbance at 530-540nm was read on a plate reader. Final results were normalized to total protein concentration as determined by Bradford assay.
From each sample 25μg of denatured protein was also separated on polyacrylamide by electrophoresis and then transferred to PVDF membranes by electrophoresis. These membranes were incubated with antibody to Hemeoxygenase-1 (Santa Cruz Biotechnology, sc-1796) for one hour at room temperature. Membranes were then washed three times and incubated with secondary antibody (donkey anti-goat HRP conjugated, Santa Cruz, sc-2056). Membranes were washed three times and bound secondary antibody detected with enhanced chemiluminesence. Resulting images were digitized and bands quantified by densitometry.
Flow Cytometry
The abdominal aortas were removed from the mice, blood content was flushed, and the aortas were minced finely and shaken in 1 ml of DMEM medium supplemented with 10% FCS, 62.5 units/ml collagenase VIII (Sigma), and 0.625 units/ml Dispase (BD Biosciences) at 37°C for 1 h. The spleen and periaortic lymph nodes were removed, cut into small pieces, and gently meshed on a tissue sieve. Cells were passed through a 70μm cell strainer and cells were counted and analyzed on a BD FACScalibur (Becton Dickenson, CA). The antibodies used to identify leukocyte subpopulations included CD45, CD3, Ly6G and f4-80. The GFP expressing cells were identified by their intrinsic fluorescence.
Leukocyte Harvest and Adoptive Transfer
Each spleen was homogenized through a 70 mm cell strainer into a sterile buffered solution of 1:50 fetal calf serum, 5mM glutamine, and 10mM glucose with Pen/Strep antibiotic and 1.5mM EDTA. Peripheral blood was collected in EDTA. Bone marrow was collected from mouse femurs and tibias where the marrow was flushed from the bone with 10ml of RPMI 1640 with fetal calf serum through a 25 gauge needle. The whole marrow is then homogenized through a 20 gauge needle and syringe. The collected cells were then centrifuged and the pellet was lysed with 3 ml of RBC lysis buffer (BioLegend, San Diego, CA). The cells were again centrifuged at 1200 rpm and 4°C for 10 minutes, and the resulting pellet was resuspended in 100 μl of buffered saline. The leukocyte preparations were combined into a 1 ml syringe. The recipient mouse was anesthetized briefly with isoflurane. The leukocyte preparation was injected into the retroorbital plexus via the outer canthus.
Electron Microscopy
Aortas were fixed by cardiac perfusion of 3% glutaraldehyde in 0.1 M sodium cacodylate (pH 7.4). Aortas were trimmed to 1.5-mm rings and treated en bloc with osmium tetroxide, tannic acid, and uranyl acetate as previously described.8 After dehydration through a graded series of methanol and infiltration with Epon, tissues were embedded in pure Epon and polymerized. Sixty-nm sections were cut and counterstained with 7% methanolic uranyl acetate and lead citrate and viewed using a Tecnai 12 transmission electron microscope at 120 kV.
Light Microscopy
Immediately following sacrifice, aortas from selected mice in each experimental group were perfusion-fixed with 10% neutral-buffered formalin, removed, and placed in fresh formalin for a minimum of 24 hr prior to processing for paraffin embedding. Sections were cut at 5 μm and mounted on glass slides. Slides were stained with hematoxylin and eosin to evaluate inflammatory cell infiltration or with Accustain® Elastic Stain kit (HT25A-1KT; Sigma-Aldrich) to assess the degree of elastin degradation. For immunohistology slides were incubated with CD5 or B220 (BD Bioscience) antibody overnight, washed and incubated with biotinylated goat anti-rat antibody followed by peroxidase-conjugated streptavidin which was detected with DAB. Photomicrographs of sections were obtained using an Olympus BX60 light microscope.
Real-time PCR and Gene Expression Analysis
Aortic tissue RNA samples were used to synthesize cDNA (Applied Biosystems; Foster City, CA). Each multiplex PCR was performed with the pre-designed TaqMan Primer and Probe with FAM dye of MMP-9 or MMP-12 (Applied Biosystems), and a custom designed primer pair and probe with VIC dye for β-actin (Forward Primer – CCCTAAGGCCAACCGTGAA, Reverse Primer – GCCTGGATGGCTACGTACATG, MGB Probe with VIC dye – ATGACCCAGATCATGT). Analysis was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems).
Statistical Analyses
The change in AD between baseline and harvest are expressed as mean percent increase from baseline (%ΔAD) ± standard error of the mean. Comparisons of the mean change in AAA size and mean protein data were analyzed using analysis of variance (ANOVA). Where multiple comparisons were required for a single experiment, false discovery was limited by the use of Tukey’s HSD. For each animal the presence of an AAA at sacrifice was defined as an increase in AD of ≥ 100% over the pre-perfusion AD. Chi-squared (or Fisher’s exact test when average cell size was less than 5) was used to test for differences in the incidence of AAAs between groups. For analysis of PCR data, ANOVA was used to calculate the mean relative expression (ΔΔCt) of the target MMP to β-actin, and the data were expressed as 2−ΔΔCt with 95% confidence intervals.9 All analyses were performed with JMP statistical software (SAS; Cary, NC) with P < 0.05 considered significant.
Results
Protease Inhibition or Deficiency Does not Prevent Enhancement of AAA by Smoking
The mean percentage change in aortic diameter (%ΔAD) at day 14 following elastase perfusion (EP) among the untreated smoke-free mice (n=20) was 126±3%. Non-specific MMP inhibition with doxycycline4 (n=9) significantly reduced aneurysmal dilation (114±5%, P<0.05).
The addition of TS exposure (n=18) prior to and following EP was associated with a significant increase in dilatation (141±4%, P=0.04) compared to the smoke-free mice. Unlike the smoke-free animals, doxycycline treatment in animals concomitantly exposed to TS (n=11) did not result in a reduction in aneurysmal dilatation. The aortic dilatation in this group was comparable to TS-exposed mice not receiving doxycycline therapy, and significantly greater than the doxycycline treated, smoke-free mice (138±8%, P<0.0001). (Fig 1A)
Figure 1. Effect of Tobacco Smoke Exposure on Aneurysmal Dilatation in Animals Treated with Doxycycline.
Doxycycline (A) is effective in inhibiting AAA development in the smoke-free animals which underwent elastase perfusion. However, when the mice were exposed to tobacco smoke regularly beginning two weeks prior to aortic perfusion, doxycycline is no longer effective in preventing the aortic dilatation. Graphs (B and C) depict the relative aortic tissue expression of MMP-9 and MMP-12 mRNA in doxycycline-treated (n=4) and untreated (n=10) mice (B) and the relative mRNA expression in doxycycline-treated mice with (n=4) and without (n=6) tobacco smoke exposure (C). Zymography (D) was performed on protein extracts from 4 aortas taken from each experimental group. There was no effect of either doxycycline or smoke exposure on the zymographic bands produced by MMP-9 or MMP-2.
Changes in expression levels of MMP-9 and MMP-12 mRNA in the aortas were measured by relative quantification RT-PCR. We have previously shown no effect of smoking alone on expression of MMP-9 or MMP-12 in this model.4 There was no significant change in MMP-9 expression in aortas with doxycycline treatment alone or with TS-exposure in doxycycline treated mice. Doxycycline treatment was associated with increased aortic MMP-12 expression, but TS-exposure in doxycycline-treated mice resulted in no significant change in expression of MMP-12 following EP (Fig 1B and 1C). Using gelatin zymography of aortic tissue extracts, proteolytic bands corresponding to MMP-2 and MMP-9 were observed for all aortic tissue specimens. There were no discernible differences in the intensity of these proteolytic bands between smoke-free and TS-exposed mice (Fig 1D).
In characterizing the structural changes that accompany AAA development, elastase-induced AAAs were found to display both elastic fiber degradation and transmural inflammatory cell infiltration (Fig 2B). These changes were exacerbated in AAAs from smoke-exposed mice, with more extensive degeneration of elastin and increased aortic wall inflammation (Fig 2C). Treatment of smoke-free mice with doxycycline resulted in preservation of elastic fibers and a reduction in inflammatory cell infiltrates (Fig 2D), however, the aortas from TS-exposed mice that had been treated with doxycycline (Fig 2E) demonstrated elastin fragmentation and medial inflammation similar to smoke-exposed animals without doxycycline treatment.
Figure 2. Increased Inflammation and Elastin Loss with Tobacco Smoke Exposure in Doxycycline Treated Animals.
Images are representative sections of aortas after EP stained with an elastin specific stain. Section (A) is from a normal, aorta. Sections (B – E) were harvested from animals 14 days after EP. In animals unexposed to TS and untreated with doxycycline, there is modest elastin loss and inflammatory cell infiltration (B). This effect is exacerbated by TS (C). When treated with doxycycline, the aortas derived from the room air exposed mice showed relatively minimal elastin damage (D). There was no effect of doxycycline on the aortic histology in the smoke-exposed mice (E).
Using animals deficient in either MMP-9 or MMP-12, we evaluated the effects of TS exposure on AAA formation. In smoke-free MMP-9 deficient C57/Bl6 mice (n=4), there was a dramatic decrease in the extent of aortic dilatation compared to wild-type animals (81±4%, P<0.0001). When MMP-9-/- animals (n=7) were exposed to TS and EP, the aortic dilatation was significantly greater (116±4%, P<0.0005), and was statistically indistinguishable from the dilatation in smoke-free, wild-type controls (Fig 3A). These effects of smoking were confirmed in 129/SvEv strain mice that were also MMP-9 deficient (Supplemental Fig S1). In smoke-free animals, the absence of MMP-12 (n=7) was associated with a significant reduction in aortic dilatation (96±2%, P<0.0001) compared to wild-type – an effect overcome by concomitant exposure to TS (n=9) resulting in significantly larger AAAs (122 ± 4%, P<0.0002) similar to those observed in wild-type mice. (Fig 3A) By histology, the exacerbation of elastin loss by smoke exposure in MMP-9 deficient animals was similar to the effect of smoke exposure on the aortas of animals treated with doxycycline (Fig 3B).
Figure 3. Effect of Tobacco Smoke Exposure on the Development of Aneurysms.
After EP, smoke exposure resulted in significantly increased AAA diameter in both MMP-9 and MMP-12 deficient mice (A). Representative sections of mouse aorta were stained with Verhoff-von Giesen (B); in smoke-free MMP-9 deficient animals, aortic elastin fibers were relatively intact with minimal inflammation, whereas, in smoke-exposed animals there was considerable elastin degradation. As with MMP elastases, the absence of either Cat-S or Neutrophil Elastase (C) did not inhibit the increased %ΔAD induced by smoke exposure. Aortic dilatation was evaluated in mice exposed to 2, 4 or 6 weeks of TS (and smoke-free controls) that underwent EP and were maintained in smoke-free conditions until harvest (D). Four weeks of TS was sufficient to enhance AAA after EP. Animals exposed to 6 weeks of TS (or 6 weeks of smoke-free conditions) were then maintained in smoke-free conditions for 0, 2, 4 or 6 weeks before EP and were harvested at day 14 (E). Remote smoke-exposure resulted in larger aneurysms in all groups. The cotinine (F) in the urine of the animals declined rapidly after smoke cessation, and was indistinguishable from smoke-free mice by 6 weeks following perfusion.
Although research in AAAs has focused primarily on the role of elastolytic enzymes of the MMP-family, serine and cysteine enzymes may also play a role.10-14 We found that smoke-free mice with targeted deletion of neutrophil elastase (NE, n=11) exhibit a significant reduction in aneurysmal dilatation following EP (98±3%, P<0.02) compared to wild-type mice. However, as with the MMP deficient mice, the AAAs that developed in the TS exposed mice (n=13) were significantly larger (133±7%, P<0.001) than the smoke-free knock-out mice. With Cathepsin-S (Cat-S) deficient mice, aneurysmal dilatation following EP in smoke-free animals (n=6) was not significantly reduced compared to WT (108±8%, P=N.S.), but concomitant TS exposure (n=9) nevertheless resulted in a significant increase in %ΔAD (147±14%, P<0.03). (Fig 3C)
Smoke Exposure Durably Enhances AAA Development
Male C57/Bl6 mice were exposed to smoke for 2 (n=4), 4 (n=6) or 6 weeks (n=6). Controls were maintained in identical housing without TS for 2 (n=14), 4 (n=6) or 6 weeks (n=7) prior to EP. All mice then underwent EP, and none of the mice were exposed to TS after EP. For reference, a separate group of 2 week TS exposed mice (n=19) continued TS after EP. We found that 4 or 6 weeks of TS exposure resulted in significantly larger AAAs at 14 days after EP than mice that had not been maintained in smoke-free conditions (Fig 3D).
To evaluate whether the effects of smoke exposure on AAA development were durable in the longer-term, experimental mice were exposed to TS for 6 weeks, then kept without TS for a smoke-free interval (SFI) of 0 (n=6), 2 (n=6), 4 (n=6) or 6 (n=5) weeks prior to EP. Smoke-free littermate controls were maintained for 6 (n=6), 8 (n=6), 10 (n=6) or 12 (n=5) weeks prior EP. Following EP, none of the mice were exposed to TS. Urine from a subset of animals was analyzed for cotinine at the time of EP or at the time of harvest.
All mice exposed to TS, regardless of the SFI, developed significantly larger AAAs at 14 days after EP than the smoke-free animals (Fig 3E). The effect did appear to become less prominent as the SFI increased following smoke cessation. The mean cotinine concentration in the urine of the animals following 6 weeks of TS was 106ng/ml, but had returned to background levels by 6 weeks after cessation of smoke exposure (Fig 3F).
Inflammation and elastin content of the harvested aortas were graded on a 0 to 4 scale by pathologists blinded to the experimental exposures of the animal. In a multivariable analysis, we found that there was no significant effect of smoking or the smoke free-interval on the degree of inflammation. Smoke exposure did significantly decrease (P < 0.04) the amount of elastin in the wall of aorta at harvest. There was no significant effect of the duration of the SFI on the degree of elastin destruction in the wall of the aorta among the smoke-exposed mice.
Effects of Tobacco Smoke on Non-Aneurysmal Aortic Tissue
To evaluate the effect of smoking alone (without EP) on the ultrastructure of the elastic fibers in the aortic wall at high resolution, three mice were TS exposed for 6 weeks, and three littermates were smoke-free controls. No other manipulations of the aortas were performed. After the 6 weeks, the animals were sacrificed and the aortas harvested and prepared for electron microscopy. In both smoke-free and smoke-exposed aortas, the elastic fibers showed a solid core of elastin with extensions projecting towards the adjacent smooth muscle cells, similar amounts of intervening extracellular matrix (ECM, such as collagen fibers), and normal appearing cell-elastin contacts (Fig 4). Importantly, the aortic walls from the two conditions were indistinguishable to a blinded observer.
Figure 4. Electron Microscopic Analysis of the Aortic Wall.
Mice were kept smoke-free or exposed to smoke for 6 weeks prior to the aortas being harvested and prepared for electron microscopy. Vessels were analysized in both cross-section (A, B) and longitudinal section (C, D) since the elastic laminae show a different appearance in the different orientations. No discernible differences were seen within the aortic walls of smoke-free (A, C) and smoke-exposed (B, D) mice with respect to elastic fiber ultrastructure, surrounding matrix or cell-elastin interactions. Bar in A = 2 μm (same for all panels).
To evaluate the effect of smoke-exposure on the oxidative stress and oxidation of the aorta, we assayed aortic tissues for Heme Oxygenase-1 by Western Blot, and performed an assay for thiobarbituric acid reactive substances (TBARS). There was no significant difference in the presence of either of these markers in the aortic tissue taken from animals after 6 weeks of smoke exposure (Supplemental Fig S2).
Leukocyte Transfer From Smoke-Exposed Animals Promotes AAA in Smoke-Free Animals
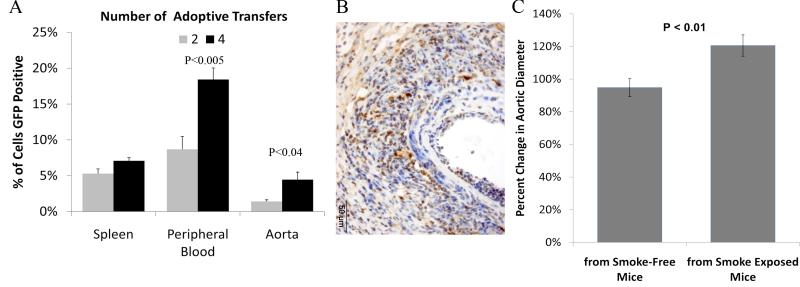
Preliminary studies were performed to determine whether adoptively transferred leukocytes would localize to the AAA wall during aortic aneurysm development. Total leukocyte populations from peripheral blood, spleen and bone marrow were harvested from somatic Green Fluorescent Protein (GFP) expressing mice. These cells were washed and adoptively transferred into wild-type smoke-free mice which underwent model AAA initiation. Transfers were conducted either 2 times (day −1 and day +3 relative to EP) or 4 times (days −5, −1, +3 and +5). At day 14 following EP, the aorta, spleen and peripheral blood of the animals were harvested.
Flow cytometry of the fresh cells derived from the spleen, aorta and peripheral blood of the mice (n=3 each) receiving adoptive transfer of cells confirmed that 4 infusions of leukocytes from GFP mice resulted in significantly more GFP+ cells in all three tissues than in the tissues from animals receiving only 2 infusions of leukocytes (Fig 5A). Paraffin embedded aortas were sectioned and immunostained for GFP. We found GFP positive mononuclear cells in both the spleen and aortic tissue (Fig 5B). There appeared to be qualitatively more cells in the 4 times transfer recipient compared to the 2 times transfer recipient mice.
Figure 5. Adoptive Transfer of Cells from Smoke-Exposed Mice Enhances AAA Development in Smoke-Free Mice.
Washed leukocyte preparations were infused from animals which constitutively express GFP into otherwise syngeneic wild-type C57/Bl6 mice. Transfers were conducted either 2 times or 4 times. At day 14 following EP, the aorta, spleen and peripheral blood of the recipient animals were harvested. By flow cytometry (A), there were significantly more GFP cells found in the aortas and peripheral blood of animals receiving 4 rather than 2 infusions of cells. Immunohistology for GFP confirmed localization of adopted cells to the aneurysm. The recipients of leukocytes from smoke-exposed mice developed significantly larger AAAs than mice which received transfers from smoke-free animals (C).
Two groups of wild-type C57/Bl6 mice were either exposed to TS for 6 weeks, or maintained in smoke-free conditions, concurrently. The leukocytes were harvested, washed and adoptively transferred using the 4 times schedule to smoke-free recipient C57/Bl6 mice which underwent model AAA initiation with EP. A recipient mouse received only transfers from either smoke-exposed or smoke-free mice for all 4 transfers. At day 14 following EP, the animals which received adoptive transfers from the smoke-exposed mice (n=10) developed AAAs which were significantly larger than the AAAs which developed in the mice which received adoptively transferred cells from smoke-free mice (n=8) (Fig 5C).
Smoke Exposure Alters the Population of Leukocytes in Model Aneurysms
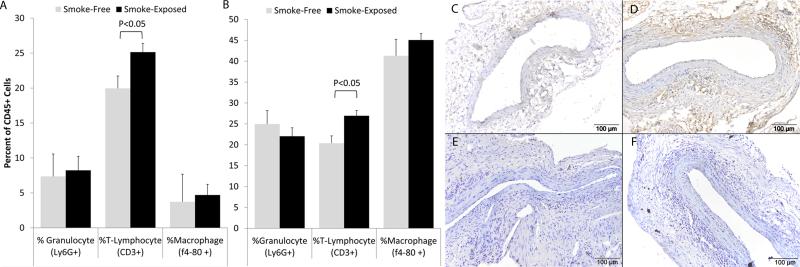
Animals were exposed to tobacco smoke (n=6) or smoke-free conditions (n=6) for 4 weeks prior to EP and continued the same exposure group until harvest at 2 weeks following EP. At the time of sacrifice, the cells of the aortas, spleens and periaortic lymph nodes were harvested and analyzed by flow cytometry for markers of leukocytes (CD45), T-cells (CD3), granulocytes (Ly-6G), and macrophages (f4-80). The total number of leukocytes constituted 3.3 ± 0.7% of the cells in the aneurysms of smoke-exposed animals and 1.4 ± 0.4% of cells in the smoke-free animals (P = 0.07). In the smoke-exposed animals, the percentage of cells that were CD3+ was significantly greater than in the smoke-free animals (Fig 6A), and we identified a similar finding the splenic tissue of these animals (Fig 6B).
Figure 6. Leukocyte populations in the wall of experimental AAA.
Aortic tissue was harvested from mice after 6 weeks of smoke exposure (or smoke-free conditions) with aortic EP two weeks prior to harvest. Flow cytometry was performed with antibodies to CD45 (total leukocytes), CD3 (T-lymphocytes), Ly6G (neutrophils) and f4-80 (macrophages). The sub-populations of leukocytes in the spleen (A) and aorta (B) are shown as a percentage of total leukocytes. Immunohistology was performed with markers for T-cells (CD5, C and D), and B-cells (B220, E and F). The T-cells were found primarily at the outer edge of the media with some cells also found within the media. The B-cells were primarily seen in the adventitia. Although an increase in total T-cell numbers in smoke-exposed animals was evident on histology, there was no difference in the localization of these cell types based on smoke (C and E) or smoke-free conditions (D and F).
Immunohistology for lymphocytes (CD5 for T-cells and B220 for B cells) was performed on sections of aortic aneurysms from smoke-exposed and smoke-free mice (Figure 6C-F). Consistent with the flow cytometry data, there is an increase in CD5 positive cells apparent in the wall of aortas taken from smoke-exposed animals. These cells were principally found in the outer media and inner adventitia and there were no differences in the localization of the lymphocytes in the aneurysm wall between smoke exposed and smoke-free animals.
Discussion
A remarkably strong clinical association exists between cigarette smoking and aortic aneurysm disease. The relative risk of developing an AAA among chronic smokers is as high as 800%, at least 2.5 times greater than the relative risk of coronary artery disease, and is exceeded only by pulmonary malignancy and emphysema.1 The clinical epidemiology of AAAs has also shown that remote exposure to TS, despite smoke cessation, can promote AAA development in subsequent years. In these studies, we have evaluated the fidelity of our novel model with the clinical disease, as well as evaluated three potential mechanisms of this effect in a novel animal model: enhanced MMP protease activity, alterations of aortic wall, and alterations of leukocyte function.
When we assessed the impact of smoking cessation on model AAA development, we found that, like the clinical disease, enhancement of AAA development is not dependent on the ongoing exposure to inhaled smoke. Moreover, after relatively modest initial exposure to TS, the enhanced development of the AAA persists even after smoke exposure has stopped and no long-lived metabolites of the exposure are detectable at the time of EP.
Based on inferences from available evidence, others have suggested in more indirect studies that increased MMP activity links smoking to AAA.15 Elastolytic metalloproteases, such as MMP-9 and MMP-12, have been of particular interest with respect to the loss of medial elastic fibers, a characteristic feature of AAAs. MMP-9 production and activity are substantially elevated in human AAA tissue, and mice with MMP-9 deficiency are resistant to the induction of experimental AAAs.7, 16, 17 MMP-12 is also elevated in human AAA tissue, where it is localized to residual elastic fibers in the media.18 In addition, MMP-12 appears to be critical for AAA development in some experimental models,19 and MMP-12 expression is upregulated in the mouse lung by TS exposure and it plays an important pathophysiologic role in smoke-induced experimental emphysema.20 Prior studies have shown that MMP inhibition or deficiency consistently suppresses the development of AAA in experimental models, including EP-induced AAAs.7, 21-24
Yet in this smoke enhanced model, we found that the suppression of AAAs with pharmacologic inhibition of MMP activity by doxycycline was overcome by concomitant TS exposure. We also found that there were no consistent changes in the expression of MMPs in the aorta that could account for the TS-induced enhancement of aneurysmal dilatation. At the protein level, we also found that aortic wall MMP-9 and MMP-2 activity was unaffected by TS exposure. Similar to our findings with doxycycline treatment, we also found that TS exposure in both MMP-9 -/- and MMP-12 -/- mice results in significantly larger AAAs. The aneurysms that developed in these animals following TS exposure were comparable in size and histologic appearance to the AAAs that occurred in wild-type controls. These findings strongly suggest that the adverse effects of TS exposure on aortic wall inflammation, ECM, and AAA development are independent of MMP activity.
Alternative enzyme classes that might participate in enhanced aortic wall injury in the setting of TS exposure include serine or cysteine proteases. A strong association has been found between cysteine proteases in both human AAAs and animal models and it has been suggested that these enzymes may work cooperatively with MMPs to damage structural ECM proteins.25 Cat-S is a particularly potent AAA associated elastase25 discovered due to its increased production and activity in response to TS exposure in the lung.26 Similarly, serine proteases, such as NE have been long thought to play a role in the elastolysis central to pulmonary emphysema in smokers.27 Recent data has also accumulated that neutrophils and NE play an important role in model AAA development.13, 14 Therefore it was surprising that neither NE nor Cat-S deficiency exhibited any suppression of model aortic dilatation in response to TS exposure. Although this finding does not exclude the possibility that other serine or cysteine proteases may mediate aortic wall damage induced by TS exposure, it reinforces the likelihood that the mechanism promoting AAA during TS exposure is distinct from that occurring during AAA development in smoke-free mice.
Because the effect of smoke-exposure was durable long after smoke cessation, we looked for alterations in the aorta induced by TS that might predispose to a greater effect of EP on AAA growth. To evaluate whether the mechanism of the smoke-enhanced AAAs was due to intrinsic changes to aortic wall structure or organization, we examined aortas from smoke-exposed and smoke-free animals by electron microscopy. No detectable changes to the aortic wall matrix (particularly the elastic fiber) or cell-matrix interactions were found when the animals were exposed to smoke alone. While others have found changes in human VSMC from aneurysms consistent with oxidative stress,28 we did not find smoke exposure alone was responsible for any increase in the amount of thiobarbituric acid reducing substances or production of Heme-Oxygenase 1, markers of oxidation/oxidative stress which have been shown to be increased in the lungs of smokers.29, 30
We also hypothesized that the effect of smoke on AAA may be due to an altered inflammatory response. Employing adoptive transfer experiments, we have been able to uniquely demonstrate that in vivo smoke-exposure of leukocytes can exacerbate aneurysm disease in a smoke-free animal. We also found the proportion of T-cells in the aneurysms of smoke-exposed mice was increased compared to smoke-free mice. Although it is possible that some tobacco related compounds might have remained with the leukocytes during transfer, it is unlikely that these would have resulted in the effects observed due to the findings that minimal exposures to TS (≤ 2 weeks) are not sufficient to cause enhanced AAA development in the absence of ongoing smoke exposure. These findings also confirm that smoke-induced alterations in the aorta itself are not necessary for the enhanced development of AAA by TS.
With these studies, the effect of TS on AAA development appears to be primarily related to altered inflammatory cell function acting to enhance matrix damage through MMP-independent pathways. There have been a number of studies which have defined alterations in immune cell function and markers in smokers or after smoke exposure in animals.31 There is evidence that smoke exposure can both enhance32-34 and inhibit immune function.35, 36 For example, in smokers, there is increased soluble intracellular adhesion molecule-1 and altered numbers of monocyte and lymphocyte populations in peripheral blood.37, 38 Others have found that in mice, smoking causes relative T-cell anergy possibly related to impaired intracellular signal transduction.39
Because of the variety of known effects of smoke exposure on modulating the function of a wide variety of immune cell types, we casted a relatively wide net with the leukocyte preparation from the donor animals to be certain that we would not miss an effect. The crude preparation of leukocytes used in the project to define this novel mechanism of aneurysm development will need to be refined in future studies. We expect that this novel model will allow us to define the alterations induced by TS in leukocytes, as well as determine the unique matrix degrading activities. More broadly, these findings suggest that the mechanisms underlying other smoking-associated inflammatory vascular diseases, such as atherosclerosis, may remain to be fully elucidated.
Supplementary Material
Acknowledgements
We are grateful to Dale Kobayashi, BA, Dept. of Pediatrics, Washington University for assistance in exposing the mice to tobacco smoke.
Funding Sources: Supported by the Flight Attendants Medical Research Institute (JAC), the American Heart Association 0765432Z (JAC), the National Heart, Lung, and Blood Institute K08 HL84004 (JAC) and P50 HL083762 (RWT), the Department of Veterans Affairs (JAC), and the Canadian Institutes of Health Research MOP86713 (ECD). ECD is a Canada Research Chair.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The health consequences of smoking: A report of the surgeon general. 2004. pp. 396–367. [PubMed]
- 2.Erhardt L. Cigarette smoking: An undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. doi: 10.1016/j.atherosclerosis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002;105:849–854. doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 4.Bergoeing MP, Arif B, Hackmann AE, Ennis TL, Thompson RW, Curci JA. Cigarette smoking increases aortic dilatation without affecting matrix metalloproteinase-9 and -12 expression in a modified mouse model of aneurysm formation. Journal of Vascular Surgery. 2007;21:328–338. doi: 10.1016/j.jvs.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 5.Stolle K, Berges A, Lietz M, Lebrun S, Wallerath T. Cigarette smoke enhances abdominal aortic aneurysm formation in angiotensin ii-treated apolipoprotein e-deficient mice. Toxicology Letters. 2010;199:403–409. doi: 10.1016/j.toxlet.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase b) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest. 1993;68:89–99. [PubMed] [Google Scholar]
- 9.Yuan J, Reed A, Chen F, Stewart CN. Statistical analysis of real-time pcr data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abisi S, Burnand KG, Waltham M, Humphries J, Taylor PR, Smith A. Cysteine protease activity in the wall of abdominal aortic aneurysms. Journal of Vascular Surgery. 2007;46:1260–1266. doi: 10.1016/j.jvs.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Xu W-H, Shi G-P, Sukhova GK, Sun J, Libby P, Yang J-T, Fu H, Ma L, Ren A, Dolganov GM, Hu C. Cathepsin l expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184:302–311. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, Upchurch GR., Jr Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 13.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, Pham CTN. Critical role of dipeptidyl peptidase i in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci U S A. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano MB, Zhou H-f, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CTN. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carty CS, Soloway PD, Kayastha S, Bauer J, Marsan B, Ricotta JJ, Dryjski M. Nicotine and cotinine stimulate secretion of basic fibroblast growth factor and affect expression of matrix metalloproteinases in cultured human smooth muscle cells. Journal of Vascular Surgery. 1996;24:927–935. doi: 10.1016/s0741-5214(96)70038-1. [DOI] [PubMed] [Google Scholar]
- 16.McMillan WD, Patterson BK, Keen RR, Shively VP, Cipollone M, Pearce WH. In situ localization and quantification of mrna for 92-kd type iv collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995;15:1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- 17.Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapiere CM. Activated forms of mmp2 and mmp9 in abdominal aortic aneurysms. Journal of Vascular Surgery. 1996;24:127–133. doi: 10.1016/s0741-5214(96)70153-2. [DOI] [PubMed] [Google Scholar]
- 18.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. Mmp-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 21.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin ii-induced atherosclerosis and abdominal aortic aneurysms. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 22.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: Preservation of aortic elastin associated with suppressed production of 92 kd gelatinase. Journal of Vascular Surgery. 1996;23:336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 23.Sho E, Chu J, Sho M, Fernandes B, Judd D, Ganesan P, Kimura H, Dalman RL. Continuous periaortic infusion improves doxycycline efficacy in experimental aortic aneurysms. Journal of Vascular Surgery. 2004;39:1312–1321. doi: 10.1016/j.jvs.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli M, Parodi F, Chu J, Pagano M, Mao D, Baxter B, Buckley C, Ennis T, Thompson R. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Annals of Vascular Surgery. 2006:1–9. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 25.Sukhova GK, Shi G-P. Do cathepsins play a role in abdominal aortic aneurysm pathogenesis? Annals of the New York Academy of Sciences. 2006;1085:161–169. doi: 10.1196/annals.1383.028. [DOI] [PubMed] [Google Scholar]
- 26.Reilly JJ, Chen P, Sailor LZ, Wilcox D, Mason RW, Chapman HA. Cigarette smoking induces an elastolytic cysteine proteinase in macrophages distinct from cathepsin l. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1991;261:L41–L48. doi: 10.1152/ajplung.1991.261.2.L41. [DOI] [PubMed] [Google Scholar]
- 27.Djekic UV, Gaggar A, Weathington NM. Attacking the multi-tiered proteolytic pathology of copd: New insights from basic and translational studies. Pharmacology & Therapeutics. 2009;121:132–146. doi: 10.1016/j.pharmthera.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C, Bianco P, Dallegri F, Pistoia V, Pezzolo A, Palombo D. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One. 2012;7:e35312. doi: 10.1371/journal.pone.0035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva Bezerra F, Valença SS, Lanzetti M, Pimenta WA, Castro P, Gonçalves Koatz VL, Porto LC. A-tocopherol and ascorbic acid supplementation reduced acute lung inflammatory response by cigarette smoke in mouse. Nutrition. 2006;22:1192–1201. doi: 10.1016/j.nut.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Wang L, Gong T, Yu Y, Zhu C, Li F, Wang L, Li C. Egr-1 regulates ho-1 expression induced by cigarette smoke. Biochemical and Biophysical Research Communications. 2010;396:388–393. doi: 10.1016/j.bbrc.2010.04.102. [DOI] [PubMed] [Google Scholar]
- 31.Sopori M, Goud N, Kaplan A. Effects of tobacco smoke on the immune system. In: Dean J, Luster M, Munson A, Kimber I, editors. Immunotoxicology and immunopharmacology. Raven Press; New York: 1994. pp. 413–434. [Google Scholar]
- 32.Winkler AR, Nocka KN, Williams CMM. Smoke exposure of human macrophages reduces hdac3 activity, resulting in enhanced inflammatory cytokine production. Pulmonary Pharmacology & Therapeutics. doi: 10.1016/j.pupt.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Koarai A, Yanagisawa S, Sugiura H, Ichikawa T, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. Cigarette smoke augments the expression and responses of toll-like receptor 3 in human macrophages. Respirology. 2012 doi: 10.1111/j.1440-1843.2012.02198.x. no-no. [DOI] [PubMed] [Google Scholar]
- 34.van Eijl S, Mortaz E, Ferreira AF, Kuper F, Nijkamp FP, Folkerts G, Bloksma N. Humic acid enhances cigarette smoke-induced lung emphysema in mice and il-8 release of human monocytes. Pulmonary Pharmacology & Therapeutics. 2011;24:682–689. doi: 10.1016/j.pupt.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Wongtrakool C, Grooms K, Ping X-D, Rivera H, Ward J, Roser-Page S, Roman J, Brown LAS, Gauthier TW. In utero nicotine exposure promotes m2 activation in neonatal mouse alveolar macrophages. Pediatr Res. 2012 doi: 10.1038/pr.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimada ALB, Ribeiro ALT, Bolonheis SM, Ferraz-de-Paula V, Hebeda CB, Farsky SHP. In vivo hydroquinone exposure impairs mcp-1 secretion and monocyte recruitment into the inflamed lung. Toxicology. 2012;296:20–26. doi: 10.1016/j.tox.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann S, Siekmeier R, Mix C, Jaross W. Even moderate cigarette smoking influences the pattern of circulating monocytes and the concentration of sicam-1. Respir Physiol. 1998;114:269–275. doi: 10.1016/s0034-5687(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 38.Luppi P, Lain KY, Jeyabalan A, DeLoia JA. The effects of cigarette smoking on circulating maternal leukocytes during pregnancy. Clin Immunol. 2007;122:214–219. doi: 10.1016/j.clim.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: Chronic exposure to cigarette smoke impairs antigen-mediated signaling in t cells and depletes ip3-sensitive ca(2+) stores. J Pharmacol Exp Ther. 2000;293:166–171. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.