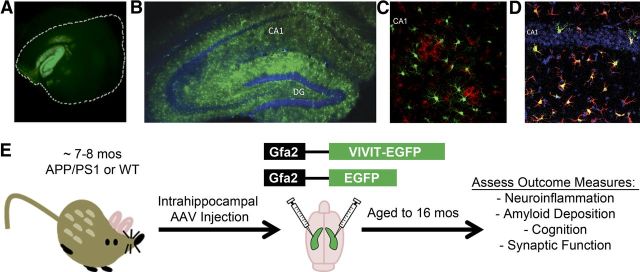
Figure 1.
AAV–Gfa2 vectors drive long-lasting and astrocyte-specific transgene expression. A–D, Representative confocal fluorescent photomicrographs showing EGFP expression in brain sections (A, longitudinal; B–D, coronal) prepared from mice that received a bilateral injection of AAV–Gfa2 vectors into the CA1 region of the hippocampal formation. At 2 months after injection (A), the longitudinal axis of the hippocampus showed abundant EGFP expression, although the neocortex, which is enclosed by the white dashed line, mostly excluded EGFP expression. At 9 months after injection (B–D), the hippocampal molecular layers, but not the dentate granule and CA1 pyramidal neuron layers (counterlabeled blue with DAPI in B and D), showed high levels of EGFP expression. Microglial cells, positively labeled for the presence of Iba-1 (red), were similarly devoid of EGFP expression (C). In contrast to neurons and microglia, numerous GFAP-positive astrocytes (red) colocalized with EGFP (green) (D), confirming that hippocampal astrocytes exclusively expressed the transgene (note EGFP/GFAP colabel appears orange/yellow). E illustrates the treatment paradigm and endpoint measures investigated in this study. WT and Tg mice received injections of either vehicle, or AAV–Gfa2 vectors containing EGFP control or EGFP coupled to the NFAT inhibitor VIVIT. We treated mice at ∼7–8 months of age, at the early stages of amyloid pathology, and then aged them to ∼16 months, at which time they underwent behavioral characterization. After the animals were killed, we assessed several AD-like biomarkers, including neuroinflammation, amyloid pathology, and hippocampal synaptic dysfunction.