Abstract
Background
Levodopa is the gold standard in the treatment of Parkinson’s disease (PD). However, long-term levodopa replacement therapy is accompanied by abnormal involuntary movements (AIMs), known as levodopa-induced dyskinesia (LID). Until now, the precise mechanisms of LID were only partially understood. Previous studies have shown that continuous dopamine stimulation was helpful in reducing the expression of LID. In addition to dopamine D1 receptor, glutamatergic receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor also contribute to the expression of LID. The current authors have previously reported that levodopa/benserazide-loaded microspheres could ameliorate the expression of LID by reducing the protein kinase A signaling pathway in dyskinetic rats. However, whether AMPA receptor is involved in the mechanism by which levodopa/benserazide-loaded microspheres ameliorate the expression of LID in dyskinetic rats was unknown.
Methods
In the present study, as reported previously, levodopa and benserazide were loaded by poly(lactic-co-glycolic acid) microspheres, which can release levodopa and benserazide in a sustained manner. 6-Hydroxydopamine was injected into the right medial forebrain bundle to produce a rat model of PD. Then valid PD rats were treated with levodopa plus benserazide for 3 weeks to induce a rat model of LID. Dyskinetic rats were treated with levodopa/beserazide-loaded microspheres containing levodopa (6 mg/kg) plus benserazide (15 mg/kg) or same dose of levodopa plus benserazide. Abnormal involuntary movements were measured in rats on days 1, 5, 10, 15, and 20 during the treatment. The levels of GluR1 at serine-831 (pGluR1S831) and serine-845 (pGluR1S845) were determined by Western blot. Arc and proenkephalin (Penk) levels were measured by real-time polymerase chain reaction.
Results
Three-week levodopa plus benserazide treatment induced dyskinesia in PD rats. Levodopa/benserazide-loaded microsphere-treated dyskinetic rats showed lower AIM scores than levodopa plus benserazide-treated dyskinetic rats. Microsphere treatment downregulated the phosphrylated levels of pGluR1S831 and pGluR1S845 in the striatum of dyskinetic rats. In addition, microsphere treatment reduced the levels of Arc and Penk.
Conclusion
These data indicated that levodopa/benserazide-loaded microspheres could be used to ameliorate the expression of LID by reducing the expression of pGluR1S831 and pGluR1S845 as well as Arc and Penk.
Keywords: levodopa/beserazide-loaded microspheres, Arc, proenkephalin
Introduction
Parkinson’s disease (PD) is a common motor neurodegenerative disease, which affects 1%–2% of the population over the age of 65.1 Levodopa therapy is the most common treatment for PD. However, about 30%–50% PD patients will show levodopa-induced dyskinesia (LID) after levodopa treatment for 5–8 years, which is a major drawback of levodopa therapy.2,3 More unfortunately, once the brain is primed to elicit dyskinesias, it is difficult to treat Parkinsonian symptoms without inducing dyskinesia. Until now, the precise mechanisms of LID have only been partially understood. It is demonstrated that pulsatile dopamine stimulation plays an important role in the expression of LID.4,5 Continuous dopamine stimulation (CDS), which can reduce the responsiveness of dopamine receptor in a direct way, is helpful in reducing LID.5 In addition to dopamine D1 receptor, previous studies have shown that glutamatergic receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, are also involved in the expression of LID.7,8 A recent study has indicated that intermittent levodopa treatment induced increased phosphorylation of AMPA in the striatum of PD rats.9 The current authors have previously reported that levodopa/benserazide-loaded microspheres, which release levodopa and benserazide in a sustained manner, could be used to reduce established LID in a rat model of PD.10 However, whether AMPA receptor is involved in the mechanisms by which levodopa/benserazide microspheres reduce LID in dyskinetic rats was still unknown. Thus, the present study was to investigate the effect of levodopa/benserazide-loaded microspheres on phosphorylated levels of GluR1, a subunit of AMPA. In addition, the effect of levodopa/benserazide-loaded microspheres on the expression of some genes related with LID such as Arc and proenkephalin (Penk) was also investigated. It was found that levodopa/benserazide-loaded microspheres reduced phosphorylated levels of GluR1 at serine-831 (pGluR1S831) and serine-845 (pGluR1S845) while ameliorating the expression of LID. In addition, levodopa/benserazide-loaded microspheres reduced the expression of Arc and Penk in dyskinetic rats.
Materials and methods
Materials
Levodopa methyl ester (LDME) and benserazide hydrochloride were obtained from Sigma-Aldrich (St Louis, MO). Dichloromethane, polyvinyl alcohol (PVA), potassium dihydrogen phosphate, phosphoric acid, and sodium chloride (NaCl) sodium phosphate dibasic anhydrous, were purchased from the Chinese Medicine Group Chemical Reagent Corporation (Shanghai, China). Methanol was purchased from Jiangsu Yonghua Fine Chemical Co, Ltd (Jiangsu, China). Poly(lactic-co-glycolic acid) 50/50 3A (47 KDa) and polylactic acid (83 KDa) were obtained from Lakeshore Biopolymers Inc (Cincinnati, OH). Water was obtained from a Milli-Q purification system (Millipore, Molsheim, France).
Animals
Ninety adult rats (Sprague-Dawley females, 180–220 g) were used in this study. All protocols involving the animals were approved by the Institutional Review Board of Xinhua Hospital and were performed according to the guidelines of the National Institutes of Health for the care and use of laboratory animals (NIH publication No 80-23).
Methods
6-hydroxydopamine (6-OHDA) lesions
6-OHDA (8 μg dissolved in 4 μL of 0.9% physiological saline containing 0.02% ascorbic acid [Sigma-Aldrich, St Louis, MO]) was stereotaxically injected into the right medial forebrain bundle of rats. The coordinates of the right medial forebrain bundle were calculated using the rat brain atlas13 as follows: anterior-posterior (AP), −4.4 mm, medial-lateral (ML), −1.2 mm, dorsal-ventral (DV), −7.8 mm. The tooth bar was set to −2.4 mm. One week after injections, the rats that exhibited a stable apomorphine-induced rotational asymmetry of at least seven full turns per minute away from the lesioned side were selected for the next experiment.
Preparation of levodopa methyl ester/benserazide-loaded microspheres
Levodopa methyl ester/benserazide-loaded microspheres were prepared as reported previously.10–12 LDME-loaded microspheres were prepared according to the oil-in-water emulsion solvent evaporation method. LDME was dissolved in distilled water to form 250 mg/mL LD solution. The solution was added to a dichloromethane solution of PLGA 50/50 3A (47 KDa, 5% w/w) and PLA (83 KDa, 7.5% w/w). The ratio of LDME to PLGA was 1:10 (w/w). The resulted water-in-oil emulsion was then added in 4 mL of a pre-cooled hydrophilic continuous phase containing 5% (w/w) PVA and 5% (w/w) NaCl at 4°C and stirred at 2000 rpm for 30 seconds using a magnetic stirrer (Sile 98-1; Shanghai Sile Co, Ltd, Shanghai, China). Once the embryonic composite microspheres were formed, the sample, including both dispersed and continuous phases, was immediately transferred into 1 L of 5% (w/v) NaCl solution at 0°C under gentle stirring (100 rpm) using an electromotive stirrer (Xinhang JJ-1; Jintan Xinhang Co, Ltd, Jiangsu, China) to extract the organic solvent and harden the embryonic microspheres. This microsphere aging process lasted 2 hours, and the hardened microspheres were then rinsed using distilled water to remove PVA and NaCl, and lyophilized to remove water and solvent residues prior to storage.
Benserazide-loaded microspheres were prepared according to the oil-in-water emulsion solvent evaporation method. Benserazide was added to the dichloromethane solution of PLGA 50/50 3A (47 KDa, 5% w/w) and PLA (83 KDa, 7.5% w/w) directly. The rest of the procedure was the same as mentioned above.
The whole process of microencapsulation was done in dark conditions to avoid light decomposition of the drugs.
Treatment
The valid PD rats were administrated with levodopa (25 mg/kg, i.p.) plus benserazide (12.5 mg/kg, i.p.) for 3 weeks to induce a rat model of dyskinesia. The dyskinetic rats (total abnormal involuntary movement [AIM] score > 8) were then divided into two groups: the LID group (n = 14) and the microsphere group (n = 14). Rats in the LID group were treated with levodopa (6 mg/kg, s.c.) plus benserazide (15 mg/kg, s.c.) daily for 3 weeks, with the assumption that levodopa/benserazide-loaded biodegradable microspheres would gradually release drug up to at least 1 week in duration. Thus, rats in the microsphere group were treated with subcutaneous levodopa/benserazide-loaded microspheres containing levodopa (6 mg/kg, s.c.) plus benserazide (15 mg/kg, s.c.) once per week for 3 weeks. Additionally, rats in the PD group (n = 14) and sham group (n = 14, given sham-6-OHDA injections without expressing PD symptoms) were treated subcutaneously, daily for 3 weeks.
AIM rating
Rats were monitored for AIMs using a procedure as reported previously.10 On test days, rats were individually placed in plastic trays 5 minutes before the drug treatments. Following injections, each rat was assessed for exhibition of axial, limb, orolingual, and locomotor movements. At 20-minute intervals (ie, 20, 40, 60, 80 minutes, etc), AIMs were rated for 60 seconds for each rat for a total of 120 minutes, during which time a severity score of 0–4 was assigned for each AIM category. Score 0 was assigned for the absence of AIMs; 1 for occasional (<50% of observation time); 2 for frequent (>50% of observation time); 3 for continuous interrupted by strong sensory stimuli; and 4 for continuous, uninterrupted AIMs. For each AIM category, the scores were summed for each time point.
Protein extraction
Total- and membrane-enriched proteins were extracted by a method described previously.14 The striatal tissue was homogenized by sonication. The homogenate was centrifuged at 800 × g for 10 minutes at 4°C, and the pellet containing mainly nuclei and large debris was discarded. The supernatant was then centrifuged again at 10,000 × g at 4°C for 30 minutes. After centrifugation, the pellet containing membrane and cytoplasmic compartments was used as total striatal protein homogenate. To pellet the synaptosomal membrane fraction, the total protein pellet was rehomogenized in ice-cold sample buffer containing 320 mM sucrose and centrifuged at 25,000 × g at 4°C for 30 minutes. After each centrifugation, the resulting pellet was rinsed briefly with ice-cold TEVP buffer before subsequent fractionations to avoid possible crossover contamination. The total striatal protein homogenate pellet and synaptosomal membrane-enriched pellet were resuspended in ice-cold sample buffer. Protein concentrations were measured by a BCA assay kit (Biyuntian, Jiangsu, China).
Western blot
Striatal tissues were homogenized (1:10, w:v) in 20 mM Tris-HCl (pH 7.4), containing 1 mM NaF, 150 mM NaCl, 1% Triton-100, and freshly added protease inhibitor cocktail (CalBiochem, La Jolla, CA), and 100 μM phenylmethylsulfonyl fluoride. Cytosols were prepared by centrifugation at 12,000 × g for 10 minutes at 4°C. Total protein (40 μg) was separated on a 10% sodium dodecyl sulphate-polyacrylamide gel and electrophoretically transferred to PVDF membranes in Tris-glycine transfer buffer. Then, membranes were blocked in 5% (w/v) instant nonfat dried milk for 2 hours at room temperature, and incubated with primary antibodies corresponding to GluR1 (1:500) (Millipore, MA), pGluR1S831 (1:250) (Millipore), or pGluR1S845 (1:250) (Millipore) at 4°C overnight. The membranes were subsequently washed with TBST (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20) and incubated with secondary horseradish peroxidase conjugated IgG for 1 hour at room temperature. Immunoreactive proteins were visualized by LumiGLO (Cell Signaling Technology, Beverly, MA) chemiluminescent reagent and peroxide. The light-emitting bands were detected with X-ray films.
Real-time PCR
After rats were sacrificed by deep anesthesia, striatal tissues of rats were homogenized, and total RNA was extracted by Trizol reagent (Life Technologies, Carlsbad, CA). The primer sequences used in this study were as follows: 5′-CTGCCACAGAAGCAGGGTGA-3′ (forward) and 5′-AGGGTGCCCACCACATACTGA-3′ (reverse) for Arc; 5′-TGGCTACAGTGCAGGCAGA-3′ (forward) and 5′-TTGTACATGTCGATGTTATCCCAAG-3′ (reverse) for Penk. The polymerase chain reaction (PCR) amplifications were performed with 50 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 20 seconds using the ABI 7300 Real-Time PCR System (Life Technologies, Carlsbad, CA). Results were expressed as relative expression corrected to the house-keeping gene β-actin.
Statistical analysis
Data were expressed as the mean ± standard deviation. Behavioral analyses were performed using a t-test. Other statistical analyses were performed using one-way analysis of variance followed by Dunnett’s t-test. P-values < 0.05 were considered statistically significant differences.
Results and discussion
Levodopa/benserazide-loaded microspheres reduced AIMs in dyskinetic rats
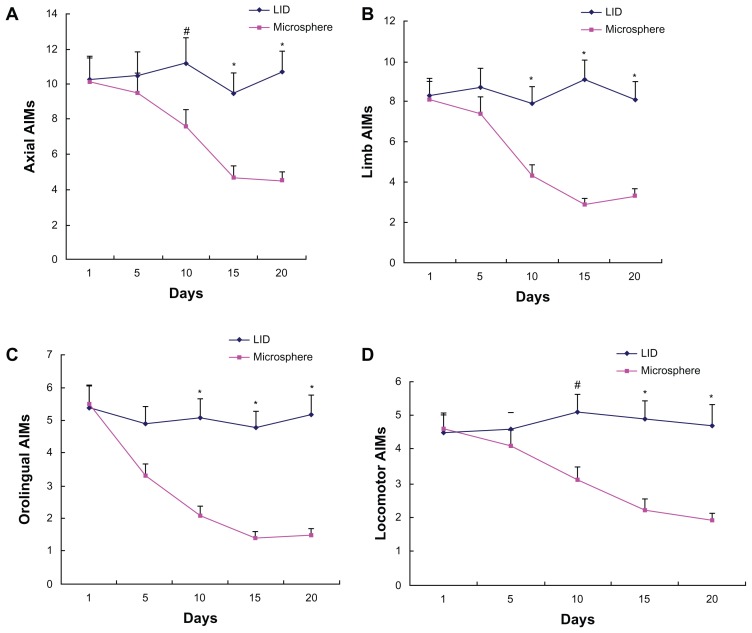
As shown in Figure 1, dyskinetic rats kept LID expression after levodopa plus benserazide treatment for 3 weeks, which was evidenced by increased AIMs. Reversely, dyskinetic rats treated with levodopa/benserazide-loaded microspheres showed decreased AIMs compared with levodopa plus benserazide-treated dyskinetic rats.
Figure 1.
Microsphere treatment reduced AIM scores in dyskinetic rats. Increased axial (A), limb (B), orolingual (C), and locomotor (D) AIM scores were found at 5, 10, 15, and 20 days in dyskinetic rats after repeated levodopa administration. However, microsphere treatment lowered AIM scores in dyskinetic rats.
Notes: Data are presented as mean ± standard error of the mean. #P < 0.05, versus microsphere; *P < 0.01, versus microsphere. The comparison was performed by t-test.
Abbreviations: AIM, abnormal involuntary movement; LID, levodopa-induced dyskinesia.
Levodopa/benserazide-loaded microspheres reduced pGluR1S831 and pGluR1S845 levels in dyskinetic rats
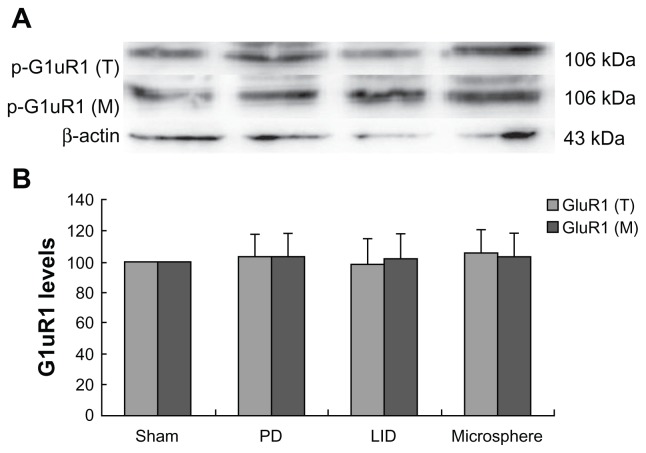
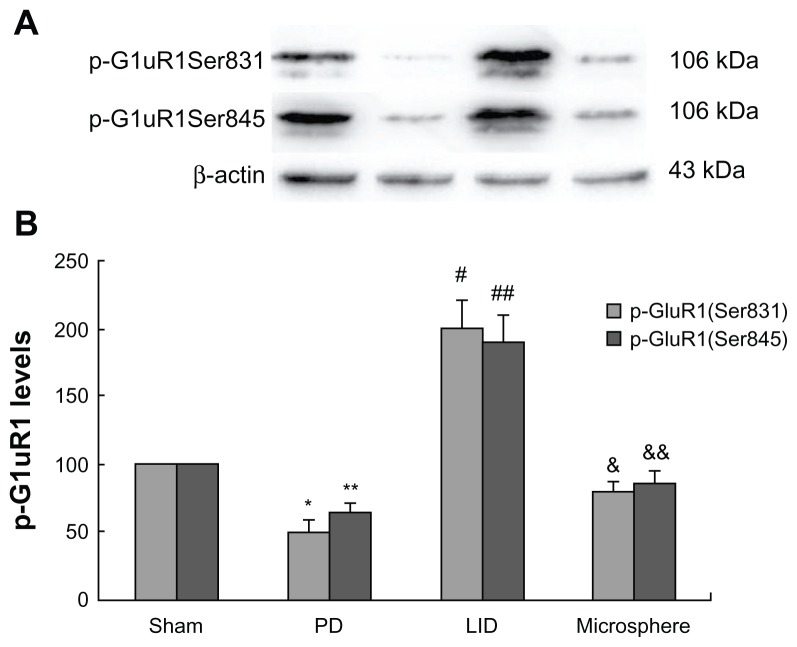
After extraction of protein from the striatum of rats, Western blot was used to determine the levels of GluR1 in the total protein and in the membrane. Additionally, pGluR1S831 and pGluR1S845 levels in the membrane were also measured. As shown in Figure 2, GluR1 levels in the total protein and the membrane were not different between different groups. However, pGluR1S831 and pGluR1S845 levels in the membrane were decreased after 6-OHDA lesions when compared with sham-operated rats (Figure 3). Repeated administration of levodopa plus benserazide increased the level of pGluR1S831 and pGluR1S845 in dyskinetic rats. However, microsphere treatment prevented the increase of pGluR1S831 and pGluR1S845 induced by intermittent administration of levodopa.
Figure 2.
Microsphere treatment had no effect on GluR1 levels in dyskinetic rats.
Notes: The band (A) represents immunoblot images detected by GluR1 in the total protein and the membrane. There was no difference in the levels of GluR1 in the total protein and the membrane between different groups. In (B), There was no difference in the levels of GluR1 in the total protein and the membrane between different groups. The comparison was performed by one-way analysis and Dunnett’s t-test.
Abbreviations: LID, levodopa-induced dyskinesia; PD, Parkinson’s disease.
Figure 3.
Microsphere treatment reduced pGluR1S831 and pGluR1S845 levels in dyskinetic rats.
Notes: The band (A) represents typical immunoblot images detected by antibodies against pGluR1S831 and pGluR1S845. pGluR1S831 and pGluR1S845 levels in the membrane were decreased in PD rats when compared with sham-operated rats. Repeated administration of levodopa increased the level of pGluR1S831 and pGluR1S845 in dyskinetic rats. Conversely, microsphere treatment reduced the increase of pGluR1S831 and pGluR1S845 induced by repeated levodopa administration. Data are presented as mean ± standard error of the mean. In (B), *P < 0.05 versus Sham; **P < 0.05 versus Sham; #P < 0.05 versus PD; ##P < 0.05 versus PD; &P < 0.01 versus LID; &&P < 0.01 versus LID. The comparison was performed by one-way analysis and Dunnett’s t-test.
Abbreviations: LID, levodopa-induced dyskinesia; PD, Parkinson’s disease.
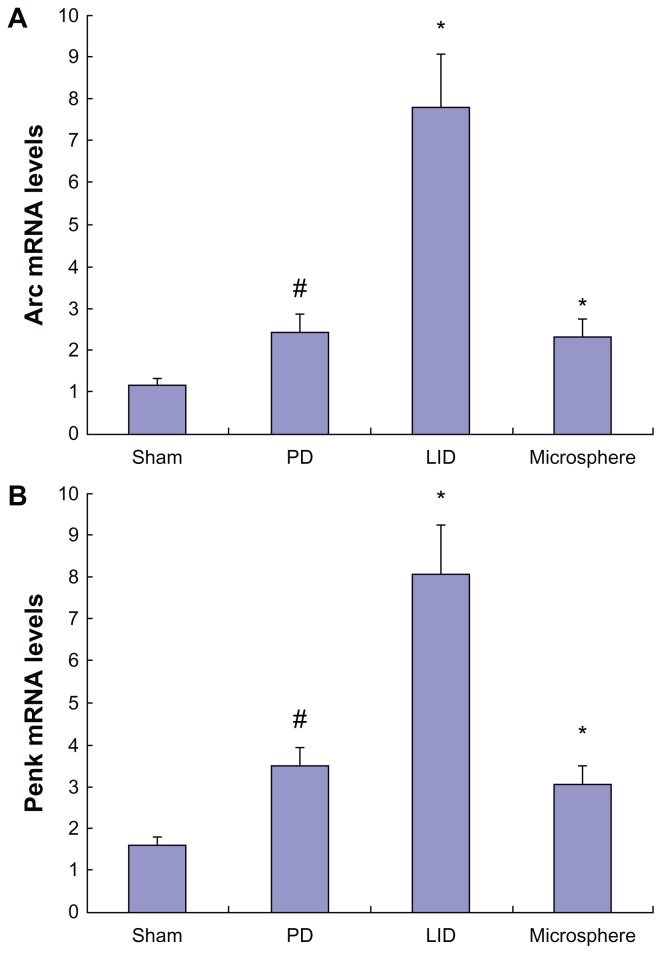
Levodopa/benserazide-loaded microspheres reduced Arc and Penk levels in dyskinetic rats
Arc and Penk levels were slightly increased after 6-OHDA lesions when compared with sham-operated rats (Figure 4). Three weeks of intermittent levodopa treatment induced a robust increase in Arc and Penk levels in dyskinetic rats. In contrast, levodopa/benserazide-loaded microspheres reduced the increase of Arc and Penk in dyskinetic rats. This indicated that intermittent levodopa treatment was helpful in the expression of Arc and Penk in dyskinetic rats.
Figure 4.
Microsphere treatment reduced the expression of Arc (A) and Penk (B) in dyskinetic rats.
Notes: Increased expression of Arc and Penk were found in PD rats. Repeated levodopa treatment further increased the levels of Arc and Penk in dyskinetic rats. Conversely, microsphere treatment reduced the expression of Arc and Penk in dyskinetic rats. Data are presented as mean ± standard error of the mean. In (A), #P < 0.05, versus Sham. *P < 0.01, versus PD. **P < 0.01, versus LID. In (B), #P < 0.01, versus Sham. *P < 0.01, versus PD. **P < 0.01, versus LID. The comparison was performed by one-way analysis and Dunnett’s t-test.
Abbreviations: LID, levodopa-induced dyskinesia; mRNA, messenger RNA; PD, Parkinson’s disease.
Discussion
Previous studies have shown that CDS is helpful in reducing the expression of LID in PD rats.15,16 More importantly, CDS could reduce established LID in dyskinetic rats.10 For the purpose of CDS, in the present study, levodopa and benserazide were encapsulated into poly(lactic-co-glycolic acid) as done previously.10 It was found that levodopa/benserazide-loaded microspheres ameliorated established LID in dyskinetic rats, suggesting that CDS was beneficial in reducing established LID in dyskinetic rats, which was consistent with a previous study.17 In agreement with the behavioral analysis, levodopa/benserazide-loaded microspheres reduced pGluR1S831 and pGluR1S845 levels while lowering AIMs in dyskinetic rats. Moreover, Arc and Penk levels were also decreased in dyskinetic rats after microsphere treatment.
The main reason for LID was that pulsatile dopamine stimulation enhanced the responsiveness of dopamine D1 receptor in the direct pathway.6,18 However, numerous studies show that in addition to dopaminergic receptor, nondopaminergic receptor such as AMPA also play an important role in the expression of LID.19–21 It is indicated that cortico-striate glutamatergic function plays an important role in the appearance of LID. Pulsatile stimulation of dopamine receptors causes plastic alterations in the synaptic efficacy of glutamatergic receptors. Repeated levodopa treatment induces changes in the phosphorylation state of AMPA receptor subunits which enhance its sensitivity to cortical glutamatergic excitation. Subsequently, the plasticity of medium spiny neuron in the striatum alters. At this moment, dopamine D1 receptor is very sensitive to dopamine stimulation. Intermittent dopamine stimulation produces over degree of phosphorylated GluR1, a subunit of AMPA, in levodopa priming PD rats.22 GluR1 can be phosphorylated at serine-831 and serine-845. The current authors previously reported that Ca2+/calmodulin-dependent protein kinase II contributes to development of LID through a mechanism that involves an increase in pGluR1S831 in PD rats.23 AMPA antagonist such as GYKI-47261 has been proven to reduce the expression of LID in animal models of PD.24 These indicate that GluR1 receptor is involved in the occurrence of LID. In the present study, it was found that pGluR1S831 and pGluR1S845 levels in the membrane of striatum were increased significantly after levodopa treatment in dyskinetic rats. However, levodopa/benserazide-loaded microsphere treatment reduced the increase of pGluR1S831 and pGluR1S845.
Previous studies show that long-term activation of PKA signaling causes the emergency of LID. It was demonstrated that repeated levodopa administration elicits profound alterations in the activity of several postsynaptic molecular markers, one of which was phosphorylated GluR1 receptor subunits, suggesting that PKA signaling was vital in the phosphorylation of GluR1 receptor.9 Furthermore, activation of PKA signaling in the direct pathway leads to increased expression of some genes such as Arc and Penk.25,26 Then dynorphin in the striatum of PD rats are increased after activation of these genes.27 More importantly, dynorphin is closely related to the expression of LID. Some agents such as glutamate metabotropic mGluR5 receptor antagonist MPEP can ameliorate LID by reducing the levels of dynorphin in PD rats.28,29 Based on these facts, it is considered that Arc and Penk are involved in the expression of LID. More importantly, PKA signaling activation is important in increasing Arc and Penk expression. In the present study, it was found that levodopa treatment induced increased levels of Arc and Penk in the striatum of dyskinetic rats, which may represent increased responsiveness of a direct pathway. However, microsphere treatment ameliorated the expression of Arc and Penk in dyskinetic rats while reducing the expression of LID. Thus, it is assumed that reduced levels of pGluR1S831 and pGluR1S845 attributed to the decreased expression of Arc and Penk after microsphere treatment in dyskinetic rats.
In conclusion, in the present study, it was found that microsphere treatment attenuated the expression of LID by inhibiting the expression of pGluR1S831 and pGluR1S845, as well as Arc and Penk in dyskintic rats.
Acknowledgments
This study was supported by the Projects of National Science Foundation of China (No 81071025 and 81171203) and Projects of the Shanghai Committee of Science and Technology, China (No 11 nm0503300, 11410708900, and 12XD1403800).
Footnotes
Disclosure
The authors declare no competing financial interests in this work.
References
- 1.Devine MJ, Gwinn K, Singleton A, Hardy J. Parkinson’s disease and α-synuclein expression. Mov Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iravani MM, Jenner P. Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm. 2011;118:1661–1690. doi: 10.1007/s00702-011-0698-2. [DOI] [PubMed] [Google Scholar]
- 3.Carta M, Bezard E. Contribution of pre-synaptic mechanisms to L-DOPA-induced dyskinesia. Neuroscience. 2011;198:245–251. doi: 10.1016/j.neuroscience.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011;69:919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- 5.Morgese MG, Cassano T, Cuomo V, Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Håkansson K, Lindskog M, Pozzi L, Usiello A, Fisone G. DARPP-32 and modulation of cAMP signaling: involvement in motor control and levodopa-induced dyskinesia. Parkinsonism Relat Disord. 2004;10:281–286. doi: 10.1016/j.parkreldis.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Kobylecki C, Hill MP, Crossman AR, Ravenscroft P. Synergistic antidyskinetic effects of topiramate and amantadine in animal models of Parkinson’s disease. Mov Disord. 2011;26:2354–2363. doi: 10.1002/mds.23867. [DOI] [PubMed] [Google Scholar]
- 8.Ouattara B, Hoyer D, Grégoire L, et al. Changes of AMPA receptors in MPTP monkeys with levodopa-induced dyskinesias. Neuroscience. 2010;167:1160–1167. doi: 10.1016/j.neuroscience.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Santini E, Sgambato-Faure V, Li Q, et al. Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS One. 2010;5:e12322. doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Zheng R, Cai Y, Liao M, Yuan W, Liu Z. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int J Nanomedicine. 2012;7:2077–2086. doi: 10.2147/IJN.S30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren T, Yuan W, Zhao H, Jin T. Sustained-release polylactide-co-glycolide microspheres loaded with pre-formulated protein polysaccharide nanoparticles. Micro and Nano Lett. 2011;6(2):70–74. [Google Scholar]
- 12.Rong X, Mo X, Ren T, et al. Neuroprotective effect of erythropoietin-loaded composite microspheres on retinal ganglion cells in rats. Eur J Pharm Sci. 2011;43(4):334–342. doi: 10.1016/j.ejps.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 2007. [Google Scholar]
- 14.Kong M, Ba M, Song L, Liu Z. Comparative effects of acute or chronic administration of levodopa to 6-OHDA-lesioned rats on the expression and phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in the striatum. Neurochem Res. 2009;34:1513–1521. doi: 10.1007/s11064-009-9939-2. [DOI] [PubMed] [Google Scholar]
- 15.Stocchi F. The therapeutic concept of continuous dopaminergic stimulation (CDS) in the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:S68–71. doi: 10.1016/S1353-8020(09)70784-9. [DOI] [PubMed] [Google Scholar]
- 16.Odin P, Wolters E, Antonini A. Continuous dopaminergic stimulation achieved by duodenal levodopa infusion. Neurol Sci. 2008;29:S387–388. doi: 10.1007/s10072-008-1054-7. [DOI] [PubMed] [Google Scholar]
- 17.Lindenbach D, Ostock CY, Eskow Jaunarajs KL, et al. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by beta-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther. 2011;337:755–765. doi: 10.1124/jpet.111.179416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaunarajs KL, Dupre KB, Steiniger A, et al. Serotonin 1B receptor stimulation reduces D1 receptor agonist-induced dyskinesia. Neuroreport. 2009;20:1265–1269. doi: 10.1097/WNR.0b013e3283300fd7. [DOI] [PubMed] [Google Scholar]
- 19.Silverdale MA, Kobylecki C, Hallett PJ, et al. Synaptic recruitment of AMPA glutamate receptor subunits in levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate. Synapse. 2010;64:177–180. doi: 10.1002/syn.20739. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord Drug Targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szénási G, Vegh M, Szabo G, et al. 2,3-benzodiazepine-type AMPA receptor antagonists and their neuroprotective effects. Neurochem Int. 2008;52:166–183. doi: 10.1016/j.neuint.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Ba M, Kong M, Ma G, et al. Cellular and behavioral effects of 5-HT1A receptor agonist 8-OH-DPAT in a rat model of levodopa-induced motor complications. Brain Res. 2007;1127:177–184. doi: 10.1016/j.brainres.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Spinnewyn B, Mautino G, Marin JG, et al. BN82451 attenuates L-dopa-induced dyskinesia in 6-OHDA-lesioned rat model of Parkinson’s disease. Neuropharmacology. 2011;60:692–700. doi: 10.1016/j.neuropharm.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Bibbiani F, Oh JD, Kielaite A, Collins MA, Smith C, Chase TN. Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD. Exp Neurol. 2005;196:422–429. doi: 10.1016/j.expneurol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Sgambato-Faure V, Buggia V, Gilbert F, Lévesque D, Benabid AL, Berger F. Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- 26.Spinnewyn B, Mautino G, Marin JG, et al. BN82451 attenuates L-dopa-induced dyskinesia in 6-OHDA-lesioned rat model of Parkinson’s disease. Neuropharmacology. 2011;60:692–700. doi: 10.1016/j.neuropharm.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Santini E, Valjent E, Fisone G. Parkinson’s disease: levodopa-induced dyskinesia and signal transduction. FEBS J. 2008;275:1392–1399. doi: 10.1111/j.1742-4658.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto N, Soghomonian JJ. Metabotropic glutamate mGluR5 receptor blockade opposes abnormal involuntary movements and the increases in glutamic acid decarboxylase mRNA levels induced by l-DOPA in striatal neurons of 6-hydroxydopamine-lesioned rats. Neuroscience. 2009;163:1171–1180. doi: 10.1016/j.neuroscience.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]