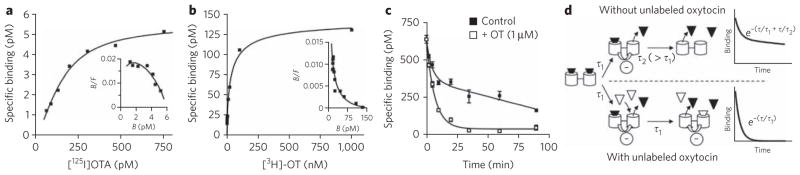
Figure 3. Binding experiments on the oxytocin receptor expressed in membrane preparations from native tissue.
(a, b) Saturation curves and the associated Scatchard plot (insets) obtained with the antagonist [125I]OTA and [3H]OT. The best fits with the Hill equation gave a Hill coefficient of 1.4 (a) and 0.54 (b). Experiments were performed on native tissues expressing oxytocin receptors (preparation of fetal membrane (a) and mammary gland preparation (b)). (c) Dissociation kinetics in the absence or presence of an excess of oxytocin; the fits correspond to a two-phase exponential decay (2.7 ± 0.3 min and 71 ± 16 min) and to a one-phase exponential decay (5.48 ± 0.59 min), respectively. (d) Diagram illustrating the influence of an excess of unlabeled oxytocin (white triangle) on the dissociation kinetics of [3H]OT (black triangle). Dissociation of receptor dimer–ligand complex follows a two-phase exponential decay with the time constants τ1 and τ2. In the absence of unlabeled ligand, owing to the negative cooperativity, the dissociation of the first ligand is faster than that of the second ligand (τ1 < τ2). The addition of an excess of unlabeled ligand does not affect the dissociation constant of the first ligand (time constant τ1) but promotes the dissociation of the second labeled ligand. Indeed, because of the negative cooperativity, the binding of unlabeled ligand on the second protomer increases the dissociation of the first labeled ligand (τ2 = τ1). Illustrated data are representative of at least three independent experiments performed in triplicate. Values correspond to the mean ± s.e.m.