Abstract
Chronic stress is a precipitating factor for affective disorders such as depression and anxiety. This is associated with the effects of chronic stress on the amygdala. Adolescents may be more vulnerable to the effects of chronic stress, which may be related to its impact on amygdala function. However, the stress-induced changes in amygdala neuronal activity, and the age-dependent impact of chronic stress on amygdala neuronal activity have not been studied in depth. In this study, we investigated how repeated restraint impacts basolateral amygdala (BLA) projection neuron activity in both adolescent and adult rats. Using in vivo extracellular recordings from anesthetized rats, we found that repeated restraint increased the number of spontaneously firing neurons in the BLA of adolescent rats, but did not significantly increase the firing rate. In contrast, repeated restraint increased the firing rate of BLA neurons in adult rats, but did not change the number of spontaneously firing neurons. This is the first direct evidence of how stress differently impacts amygdala physiology in adolescent and adult rats. These findings may shed light on the mechanism by which chronic stress may age-dependently precipitate psychiatric disorders.
Keywords: chronic stress, repeated restraint, adolescent, amygdala, extracellular electrophysiology
1
Chronic stress can induce changes in affective behaviors, including emotion and associative learning (Shors, 2004; Teicher et al, 2006). Chronic stress is associated with the development of affective disorders such as anxiety and depression (Heim and Nemeroff, 2001; Hammen, 2005). The amygdala, in addition to playing a pivotal role in processing emotional information, modulates the stress responses (Davis et al, 1994; Feldman et al, 1995; Herman and Cullinan 1997; Van de Kar and Blair 1999). However, the amygdala itself undergoes structural changes when subjected to chronic stress, such as increased dendritic branching and increased number of spines in BLA projection neurons (Vyas et al, 2002; Mitra et al, 2005). This modification of amygdala structural properties is accompanied by increased emotional reactivity (Wood et al, 2008) and enhanced amygdala-dependent affective behaviors, such as fear conditioning to discrete cues (Conrad et al, 1999; Toledo-Rodriguez and Sandi, 2007) as well as enhanced anxiety-like behaviors (Conrad et al, 1999) in experimental animals. Human imaging studies indicate amygdala hyperactivity and hyper-responsiveness in people that experienced chronic stress, such as combat veterans and abused women and children (Rauch et al, 2000; Protopopescu et al, 2005; Bremner et al, 2008), as well as patients with major depression (Drevets et al, 1992; Frodl et al, 2002). All this evidence suggests that chronic stress contributes to abnormal affective behaviors and possibly psychiatric disorders via its effect on the amygdala. The enhanced emotional reactivity after chronic stress may be driven by increased neuronal activity of projection neurons, the main efferent neurons of the amygdala. While there is much known about the impact of acute stressors on BLA physiology (e.g Shors, 1999; Vouimba et al, 2004, 2006; Pelletier et al, 2005; Kavushansky et al, 2006; Isoardi et al, 2007; Karst et al, 2010), much less is known about the effects of chronic stressors on BLA physiology. Several studies indicate that chronic stress, stress exposure repeated at least 3 times, or chronic treatments that may mimic the effects of stress, lead to increased amygdala neuronal activity through mechanisms that include increased excitability, reduced inhibition, and inappropriate modulation by monoamines (Braga et al, 2004; Correll et al, 2005; Buffalari and Grace, 2009; Jiang et al, 2009; Patel et al, 2009; Mozhui et al, 2010; Rosenkranz et al, 2010). Understanding how chronic stress affects amygdala neuronal physiology will shed light on the pathophysiology of stress-induced psychiatric disorders.
Adolescence is a critical period for brain development, characterized by neuro-anatomical rearrangements (Spear, 2000; Sisk and Foster, 2004; Romeo et al, 2006). In humans, adolescence is accompanied by an increased incidence of affective disorders (Kessler et al, 2001; Merikangas et al, 2010). Given the large effect of stress hormones on brain development, it is not surprising that brain regions undergoing maturation, such as the amygdala, are susceptible to stress during adolescence. Adolescent rodents display greater body weight loss and reduction of open arm exploration in the elevated plus maze (EPM) as well as greater cue specific fear conditioning in response to different stressors compared to adult rodents (Stone and Quartermain, 1997; Toledo-Rodriguez and Sandi, 2007). Stress exposure during adolescence may cause greater physiological disturbances than exposure during adulthood, which may lead to age-dependent differences in affective behaviors. In this study, repeated restraint was used to model the effects of chronic stress. We hypothesized that chronic stress exerts greater effect on amygdala neuronal activity in adolescent rats compared to adult rats. Uncovering the age-dependent effect of chronic stress on amygdala neuronal activity will increase our understanding of the age-dependent impact of stress on psychiatric conditions that involve abnormal amygdala function, and may produce age-appropriate preventative and curative measures for stress-induced psychiatric disorders.
Within the amygdala, BLA is the major afferent interface that receives information from all sensory modalities (Turner and Herkenham, 1991; McDonald, 1998). Previous work done in our lab has shown that repeated restraint resulted in BLA projection neuron hyper-excitability in adult rats, which may lead to increased activity of projection neurons (Rosenkranz et al, 2010). In this study, we used in vivo extracellular electrophysiological recordings to test if repeated restraint exerts a greater impact on BLA projection neuron activity in adolescent rats compared to adult rats.
2 EXPERIMENTAL PROCEDURES
2.1 Ethical Approval
All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science.
2.2 Materials
Urethane, cresyl violet and sucrose were purchased from Sigma (St. Louis, MO). Pontamine Sky Blue was purchased from Alfa Aesar (Ward Hill, MA). NaCl and formaldehyde were purchased from Fisher Scientific (Pittsburgh, PA).
2.3 Animals and Stress Protocol
Male Sprague Dawley rats (Harlan, Indianapolis, IN) arrived at postnatal day (PND) 21 and PND 53–58 for adolescent and adult rats respectively. They were housed 2 or 3 per cage in the Rosalind Franklin University animal facility with free access to food and water, and maintained on a 12 hr light/dark cycle. To model the effects of chronic stress, a 7-day repeated restraint protocol was used. Animals of the same age were randomly assigned into 4 groups: non-restraint group, two different 1-day restraint groups, and the repeated restraint group. After habituating to the animal facility for at least 4 days, rats were subjected to stress or control handling. Rats in the repeated restraint group were placed into a hemi-cylinder restraint tube 20 min/session, 1 session/day for 7 out of 9 days in the procedure room (Rosenkranz et al, 2010). The restraint tube was an acrylic cylinder with flattened bottom (dimensions dependent on animal size: rats 30–125g were placed in a cylinder 5″ × 2″, rat 125–250g in a cylinder 6″ × 2.5″, and rats 250–500g in a cylinder 8″ × 3.25″). Rats in the non-restraint group were placed into a clear Plexiglas transportation cage 20 min/session, 1 session/day for 7 out of 9 days. All the procedures were performed between 8:00 am to 3:00 pm, during the lights on cycle. To assess the additive nature of repeated restraint, we also included two control 1-day restraint groups. Rats in 1-day restraint B group (B ~ 1 day Before the behavior test) were handled the same way as non-restraint rats except they were subjected to restraint on the last day of this procedure. Rats in 1-day restraint F group (F ~ First day of the restraint protocol) were subjected to restraint on the first day of the procedure and then handled identically to non-restraint rats during the remaining 8 days.
2.4 Elevated Plus Maze
To validate the effectiveness of our repeated restraint protocol, we tested animals in the EPM one day after the final restraint/control handling session. Two sets of EPMs designed specifically for animals of different ages were used in this study. The EPM (Scientific Designs, Pittsburgh, PA) consisted of four arms: two open arms (width × length: small maze 4″ × 15″; big maze 5″ × 20″) and two closed arms (width x length x wall height: small maze 4″ × 15″ ×14″; big maze 5″ × 20″ × 18″). Each arm was attached to a sturdy leg, elevated 32 from the ground. Animals were placed at the junction of four arms, facing the open arm opposite the experimenter. Animal behavior was recorded for 5 min and analyzed by a personal computer (Dell E6500) running video-tracking software (Any-Maze, Stoelting, Wood Dale, IL). The time spent on open arms was measured and used as index of anxiety-like behavior. In addition, the number of closed arm entries was measured and used as an indicator of locomotor activity.
2.5 In vivo Extracellular Recording
To examine the neuronal activity of BLA projection neuron, we used in vivo extracellular electrophysiological recording. One day after the EPM behavioral test, rats were anesthetized with urethane (1.5 g/kg dissolved in 0.9% saline, i.p.) and placed on a stereotaxic device (Stoelting, Wood Dale, IL). Their body temperature was monitored via a rectal temperature probe, and maintained at 36–37°C using a heating pad with a temperature controller (Model TC-1000, CWE Inc, Ardmore, PA). The amygdala was localized using a stereotaxic atlas (Paxinos and Watson, 1998). The coordinates used for amygdala centered on 4.8mm – 5.5mm lateral from midline, 2.5mm – 3.8mm caudal from bregma for adult rats. Coordinates were adjusted for adolescent rats according to the measured distance between bregma and lambda. Burr holes were drilled on the skull bilaterally at locations overlying the BLA. The left hole was used for fixing a screw for electroencephalogram (EEG) recording. The dura from the right hole was removed. Single-barrel electrodes were constructed from glass pipettes (World Precision Instruments, Sarasota, FL), and pulled using a vertical microelectrode puller (PE-2; Narishige, Tokyo, Japan), and broken under a microscope to produce a tip 1 to 2 μm in diameter. The electrode was lled with 2% Pontamine Sky Blue in 2 M NaCl and then slowly lowered into the amygdala via a hydraulic microdrive (Model MO-10, Narishige, East Meadow, NY). Recordings began no earlier than 45 min after surgery.
During extracellular recording, signals were amplified by a headstage (Dagan, Minneapolis, MN) connected to a preampli er (Dagan, Minneapolis, MN), filtered at 0.3 Hz (low cut-off frequency) and 3 kHz (high cut-off frequency), and outputted simultaneously to an oscilloscope (Model 2532 BK Precision, Yorba Linda, CA) and an audio monitor (Model AM8 Grass Instruments, West Warwick, RI). In addition, amplified outputs were digitized through an interface (5–10 kHz; Model ITC-18, HEKA, Bellmore, NY) and fed to a personal computer (Mac Pro/2.8 Apple, Cupertino, CA), monitored using Axograph × software and stored on a hard disk for off-line analysis.
Throughout the experiment, the anesthetic state of the animal was monitored via cortical EEG. The EEG signal was visually inspected. Animals were considered under deep anesthesia when the EEG displayed a rhythmic waveform. Occasionally, periods of fast irregular oscillation of the EEG waveform were observed. Single unit recordings were not included for analysis if recordings occurred during this type of EEG activity. General EEG periodicity was measured by counting the number of EEG slow waves per second.
BLA projection neurons were included in analysis if they met the following criteria: First, they had to be located within the confines of the BLA, as determined by reconstruction based on histological staining. BLA, and the subnuclei of the lateral nucleus (LAT) and basal nucleus (BL) were delineated in cresyl violet-stained sections based on the borders defined in a stereotaxic atlas (Paxinos and Watson, 1998). Second, the spikes they generated had a clear signal to noise ratio (>3:1). Stable activity of projection neurons was recorded for 5 min. The activity of neurons in the BLA was expressed by the basal firing rate, quantified as the average number of spikes per second (Hz). The activity was also expressed by the number of spontaneously active neurons recorded per electrode track, a gross estimation of the relative numbers of active neurons.
2.6 Adrenal Gland
After electrophysiological recording, rats were decapitated and both adrenal glands were removed. Adrenal glands were weighed while still wet, and the weight was normalized to the animals body weight (mg/g).
2.7 Histology
At the end of electrophysiological recording, the position of the electrode tip was marked by passing a constant −25 μA current through the electrode for 20 min to eject Pontamine Sky Blue at the recording sites. Rats were immediately decapitated and their brains were removed and stored in 4% formaldehyde in 0.1 mol/L phosphate buffer overnight, and then cryoprotected in 25% sucrose in 0.1 mol/L phosphate buffer. Brains were then sliced into 60 μm thick sections using a freezing microtome (Leica Microsystems Inc, Buffalo Grove, IL) and stained with cresyl violet. Recording sites were verified by light microscopy.
2.8 Statistical Analysis
Based on analysis of preliminary data of neuronal firing rate (effect size >0.4 Hz, sigma=0.5), a sample size of 20 neurons/group would yield power of >0.8 at an alpha of 0.05. Statistical tests were performed using Prism 5 software (GraphPad, La Jolla, CA). Parameters being analyzed included: time spent on the open arm of the EPM, total number of closed arm entries of the EPM, adrenal gland weight normalized to body weight, firing rate and the number of spontaneously firing neurons per electrode track. To examine the impact of repeated restraint on behavior, endocrine function and neuronal activity of experimental rats, all the above parameters were compared between repeated restraint group and age-matched non-restraint control group and single restraint groups using one-way Analysis of Variance (ANOVA) tests followed by post hoc Newman-Keuls multiple comparison tests, or using the Kruskal-wallis test followed by Dunn s multiple comparison tests if the data were not normally distributed. The frequency distribution of values for the number of spontaneously firing neuron per electrode track was compared between groups using a Chi-square test. The cumulative distribution of firing rates was compared between groups using an F-test to compare the best-fit parameters of a second-order polynomial fit to the cumulative histograms and Kolmogorov-Smirnov (K-S) tests. To assess whether a single restraint had long lasting effect, the same parameters were also compared among the non-restraint and two age-matched 1-day restraint groups using one-way ANOVA tests, or using the Kruskal-wallis test if the data were not normally distributed. When comparing two groups in specified instances, the Mann-Whitney U test was used. A p value < 0.05 was considered statistically significant. All data were presented as mean ± SEM, unless otherwise specified.
3 RESULTS
3.1 Repeated restraint results in behavioral and endocrine changes consistent with chronic stress
To evaluate the effectiveness of the repeated restraint protocol as a stressor in both adolescent and adult rats, we examined its effect on anxiety-like behavior and a measure of endocrine function, two measures that are sensitive to chronic stress (Márquez et al, 2004; Vyas and Chattarji, 2004).
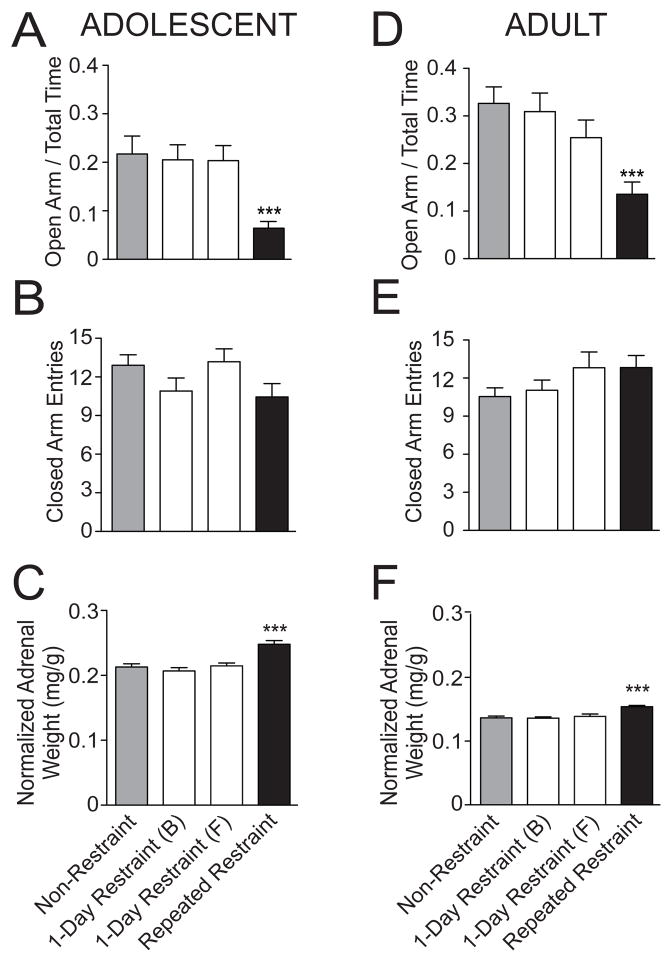
Repeated restraint caused a decrease of exploration in the open arm of the EPM when comparing the repeated restraint group to their age-matched non-restraint group or single restraint groups in adolescent rats (Fig. 1A; adolescent percentage of time on open arm: non-restraint 21.72 ± 3.70%, n=21 rats; 1-day restraint (B) 20.52 ± 3.09%, n=21 rats; 1-day restraint (F) 20.35 ± 3.10%, n=17 rats; repeated restraint 6.40 ± 1.40%, n=25 rats; p=0.0002, one-way ANOVA, F(3,80)=7.3). Post hoc analysis of the adolescent rats indicated that there was a significant difference in percentage of time on open arm between repeated restraint stress and non-restraint control groups (p<0.05, q=5.62, post hoc Newman-Keuls multiple comparison test), and both single restraint control groups (1-day restraint (B), p<0.05, q=5.18; 1-day restraint (F), p<0.05, q=4.82, post hoc Newman-Keuls multiple comparison test). However, there was no significant difference in the total number of closed arm entries among 4 treatment groups, indicating little effect of restraint on overall locomotor activity in adolescent rats (Fig. 1B; adolescent closed arm entries: non-restraint 12.90 ± 0.81, n=21 rats; 1-day restraint (B) 10.90 ± 1.02, n=21 rats; 1-day restraint (F) 13.18 ± 1.00, n=17 rats; repeated restraint 10.44 ± 1.04, n=25 rats; p>0.05, F(3,80)=1.97, one-way ANOVA).
Figure 1. Repeated restraint is an effective chronic stressor in adolescent and adult rats.
(A) Adolescent rats exposed to repeated restraint displayed less time spent on the open arm of the EPM compared to non-restraint rats, indicative of increased anxiety-like state. Single restraint did not significantly impact time spent on the open arm of the EPM. (B) There was no significant effect of repeated restraint or single restraint on the total number of closed arm entries, indicative of no effect of restraint on general locomotor activity in the EPM. (C) Adolescent rats exposed to repeated restraint displayed greater normalized adrenal gland weight compared to non-restraint rats. Single restraint did not significantly impact adrenal gland weight. (D) Adult rats exposed to repeated restraint displayed less time spent on the open arm of EPM compared to non-restraint rats. Single restraint did not significantly impact EPM exploration in adult rats. (E) There was no significant difference in the total number of closed arm entries between non-restraint and repeated restraint adult rats. Similarly, single restraint had no effect on closed arm entries. (F) Adult rats exposed to repeated restraint displayed greater normalized adrenal gland weight compared to non-restraint rats. Single restraint did not significant impact adrenal gland weight in adult rats. *** indicates p<0.001 versus non-restraint group.
Similarly, in adult rats there was also a decrease in exploration of open arms after repeated restraint (Fig. 1D; adult percentage of time on open arm: non-restraint 32.66 ± 3.47%, n=35 rats; 1-day restraint (B) 30.92 ± 3.90%, n=25 rats; 1-day restraint (F) 25.45 ± 3.68%, n=17 rats; repeated restraint 13.54 ± 2.58%, n=35 rats; p=0.0001, F(3,108)=7.7, one-way ANOVA). Post hoc analysis of these adult rats indicated that there was a significant difference in percentage of time on open arm between repeated restraint stress and non-restraint control groups (p<0.05, q=6.28, post hoc Newman-Keuls multiple comparison test), and between repeated restraint stress and both 1-day restraint control groups (1-day restraint (B), p<0.05, q=5.21; 1-day restraint (F), p<0.05, q=3.12, post hoc Newman-Keuls multiple comparison test). This is consistent with increased anxiety-like behavior after repeated restraint in both age groups. There was no significant difference in the total number of closed arm entries among 4 treatment groups, indicating little effect of restraint on overall locomotor activity in adult rats (Fig. 1E; adult closed arm entries: non-restraint, 10.54 ± 0.69 arm entries, n=35 rats; 1-day restrant (B) 11.04 ± 0.81, n=25; 1-day restraint (F) 12.82 ± 1.23, n=17; repeated restraint 12.83 ± 0.96, n=35 rats; p>0.05, F(3,108)=1.81, one-way ANOVA).
In addition, repeated restraint caused greater normalized adrenal gland weight compared to non-restraint or 1-day restraint control groups in adolescent rats (Fig. 1C; adolescent non-restraint 0.213 ± 0.005 mg/g, n=30 rats; 1-day restraint (B) 0.207 ± 0.005, n=11; 1-day restraint (F) 0.215 ± 0.005, n=31; repeated restraint 0.248 ± 0.006 mg/g, n=40 rats; p<0.001, F(3,108)=12.0, one-way ANOVA). Post hoc analysis in these adolescent rats indicated that the adrenal gland weight from the repeated restraint group was significantly greater than non-restraint controls (p<0.05, q=6.86, Newman-Keuls multiple comparison test) and single restraint groups (1-day restraint (B) p<0.05, q=5.68; 1-day restraint (F) p<0.05, q=6.56, Newman-Keuls multiple comparison test). A similar effect was found in adult rats (Fig. 1F; adult non-restraint 0.137 ± 0.003 mg/g, n=35 rats; 1-day restraint (B) 0.136 ± 0.002, n=13 rats; 1-day restraint (F) 0.139 ± 0.004, n=23 rats; repeated restraint 0.154 ± 0.002 mg/g, n=41 rats; p<0.001, F(3,108)=11.2, one-way ANOVA). Post hoc analysis in these adult rats indicated that the adrenal gland weight from the repeated restraint group was significantly greater than non-restraint controls (p<0.05, q=7.18, Newman-Keuls multiple comparison test) and single restraint groups (1-day restraint (B) p<0.05, q=5.39; 1-day restraint (F) p<0.05, q=5.56, Newman-Keuls multiple comparison test). This is consistent with repeated hypothalamic–pituitary–adrenal (HPA) axis activation in response to repeated restraint and provides support for the effectiveness of repeated restraint stress in adult and adolescent rats.
While there was significant difference between repeated restraint stress and non-restraint or single restraint groups, there was no significant difference between the non-restraint groups and the single restraint groups in adolescent or adult rats in EPM exploration (Fig. 1A; percentage of time on open arm: adolescent p>0.05, F(2,56)=0.05; adult p>0.05, F(2,74)=0.82, one-way ANOVA). Similarly, there was no significant difference in the normalized adrenal gland weight among the three control groups in adolescent rats (Fig. 1C; p>0.05, F(2,69)=0.36, one-way ANOVA test) or adult rats (Fig. 1F; p>0.05, F(2,68)=0.18, one-way ANOVA test). Therefore, a single restraint did not significantly impact EPM exploration or adrenal gland weight in adolescent or adult rats, and is unlikely to account for the effects of repeated restraint sessions. Thus, repeated restraint resulted in increased anxiety-like behavior and adrenal gland weight in both adolescent and adult rats and is an effective chronic stressor.
3.2 Repeated restraint increases the number of spontaneously firing BLA neurons in adolescent rats
BLA neurons in adolescent rats
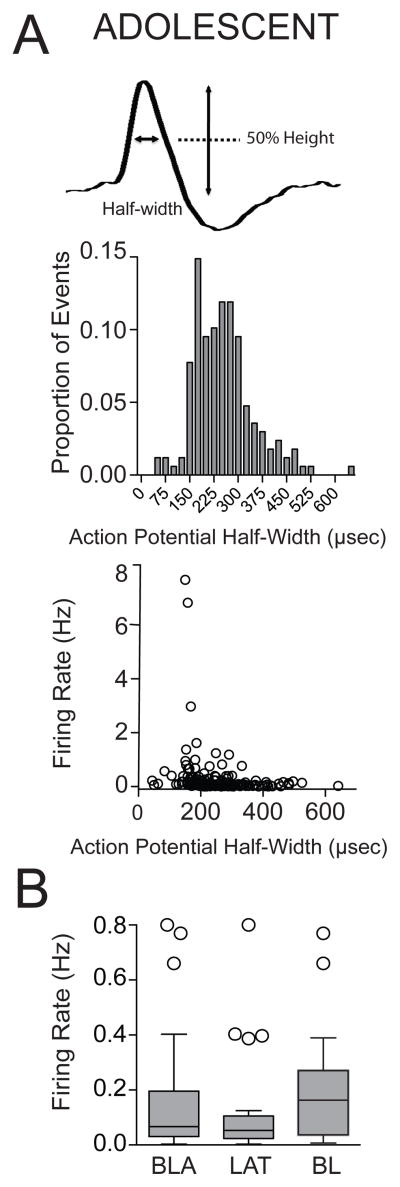
We examined how repeated restraint changes neuronal activity of BLA putative projection neurons using in vivo extracellular recordings. A total number of 47 adolescent rats were included in this study (non-restraint n=13 rats; 1-day restraint (B) n=11 rats; 1-day restraint (F) n=12 rats; repeated restraint n=11 rats). A total number of 180 neurons were confirmed to lie within the BLA. The action potential half-width showed a normal distribution (Fig. 3A; range: 30.07 to 641.46μsec). Previous studies have shown that some putative BLA interneurons display shorter action potential duration compared to projection neurons (e.g. Rosenkranz and Grace, 1999). Based on those studies, a half-width of 100μsec was used as a cut-off, and 6 neurons were omitted from analysis (non-restraint n=40 neurons; 1-day restraint (B) n=43 neurons; 1-day restraint (F) n=34 neurons; repeated restraint n=57 neurons). In line with previous research (Rosenkranz and Grace, 1999), the firing rate of spontaneously spiking neurons in BLA was very low (0.16 ± 0.03 Hz, n=40 neurons, range: 0.003–0.8 Hz) under non-restraint conditions. There was no significant difference in the firing rate between neurons from the LAT and from the BL under non-restraint conditions (Fig. 3B; LAT: 0.13 ± 0.04 Hz, n=21 neurons; BL: 0.20 ± 0.05 Hz, n=19 neurons; p>0.05, U=148.5, Mann-Whitney U test). Therefore, they were combined for initial analysis.
Figure 3. Firing of BLA neurons in adolescent rats.
(A) Enlargement of an action potential recorded from a BLA neuron showing the action potential half-width (top). Distribution histogram of action potential half-width of neurons in the BLA (middle), and plot of action potential half-width by firing rate (bottom). (B) There was no significant difference in the firing rate between neurons from the LAT and neurons from the BL in adolescent rats under non-restraint conditions. Box and whisker plots here and in all figures display interquartile range (IQR), the line represents the median, and individual data points are further than 1.5 × IQR.
Adolescent rats: Effect of repeated restraint on firing rate of BLA neurons
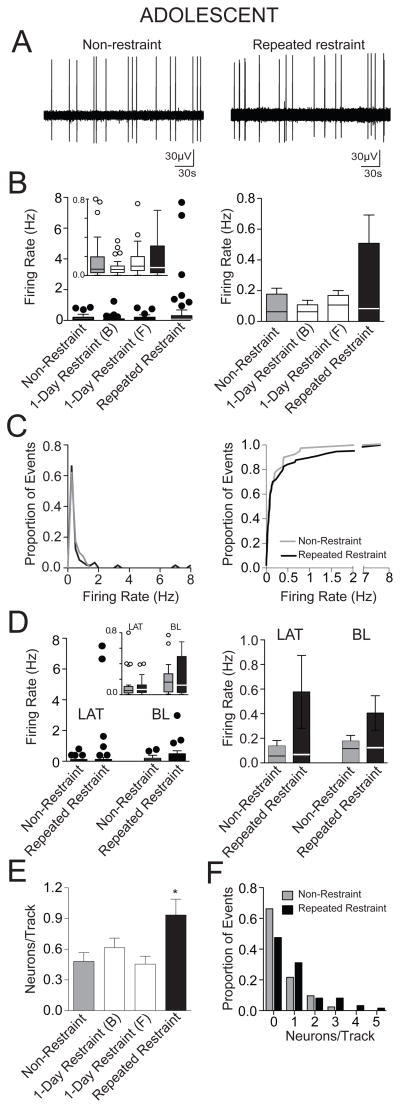
Adolescent rats exposed to repeated restraint showed no significant difference in the firing rate of BLA neurons compared to non-restraint rats and 1-day restraint control rats (Fig. 4B; adolescent non-restraint: 0.16 ± 0.03 Hz, n=40 neurons; 1-day restraint (B) 0.11 ± 0.03 Hz, n=43 neurons; 1-day restraint (F) 0.17 ± 0.03 Hz, n=34 neurons; repeated restraint 0.51 ± 0.18 Hz, n=57 neurons; p=0.31, H(3)=3.57, Kruskal-Wallis test). However, the group means clearly appeared different. For this reason, we explored this issue with further analysis. Upon closer examination it was apparent that after repeated restraint, there was a small subset of neurons that exhibited a high firing rate that skewed the group mean firing rate (1.62Hz, 2.98Hz, 6.83Hz, 7.68 Hz, mean 4.78 ± 1.47 Hz, n=4), which was absent from all control groups. The action potential half-width of these neurons were greater than 100 μsec, therefore, they could not be excluded based on that criteria. Though these neurons skewed the adolescent group means, the group medians were still close together (Fig. 4B; median: non-restraint 0.07 Hz; 1-day (B) 0.06 Hz; 1-day (F) 0.10 Hz; repeated restraint 0.08 Hz). We then tested whether the distribution of firing rates in the adolescent repeated restraint group can be better fit with a bimodal distribution or a unimodal distribution. The data were fit significantly better with a one phase decay model (r2=0.99) than a bimodial distribution (fourth-order polynomial model, r2=0.69; Aikake informative criteria difference=122.8, p<0.01), consistent with one population of neurons with a skewed distribution. Furthermore, the cumulative frequency distribution histogram of the firing rate demonstrated a similar distribution between the non-restraint group and repeated restraint group, and the distribution was not significantly different (Fig. 4C; p=0.64, F(3,81)=0.56, F-test comparison of parameters of best fit to second order polynomial; p=0.94, D=0.1066, K-S test). These analyses did not support a significant difference between the non-restraint control and the repeated restraint groups. Therefore, a conservative approach was used, and these neurons were retained in the data. However, it is important to note that if the neurons were excluded from analysis, the difference between the two groups is even less, and there is still no significant difference in the firing rate between non-restraint and repeated restraint groups (non-restraint: 0.16 ± 0.03 Hz, n=40 neurons; repeated restraint: 0.19 ± 0.04 Hz, n=53 neurons; p>0.05, U=1038, Mann-Whitney U test).
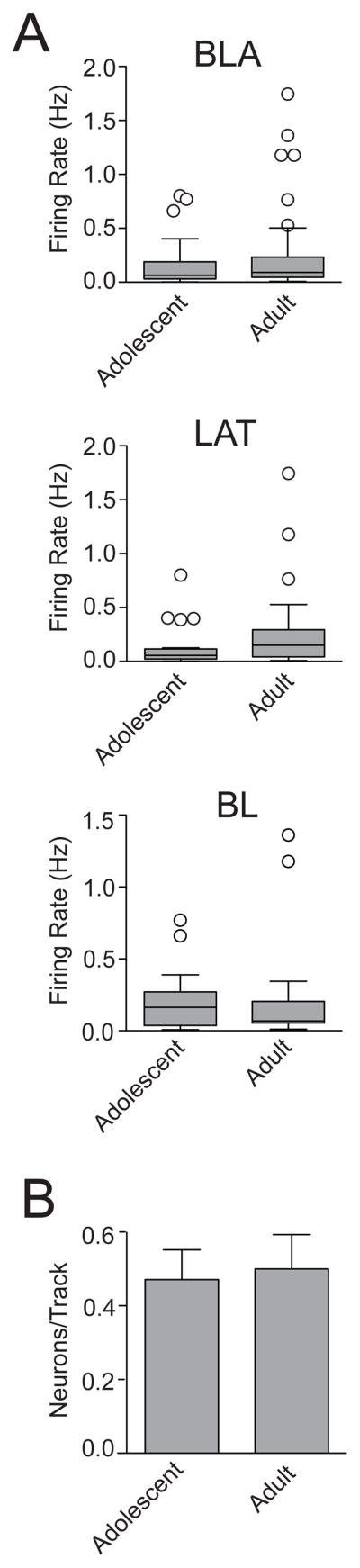
Figure 4. Repeated restraint stress increased the number of spontaneously active BLA neurons in adolescent rats.
(A) A recording trace of a BLA neuron recorded from a non-restraint rat (left) and a repeated restraint rat (right). (B) There was no significant difference in the firing rate between non-restraint and repeated restraint rats. Single restraint did not significantly impact firing rate. A box and whisker plot displays the median and 1.5 × IQR (left), with an inset of the same plot at a different scale (y-axis is cut-off at 0.8 Hz to facilitate comparison of the distribution and median in this inset). The overall mean (bar) and medians (line through bar) are displayed to facilitate comparison (right). (C) The distribution of the firing rates (left) demonstrates that there is a very similar distribution between non-restraint and repeated restraint groups. Cumulative frequency histograms display similar distribution of firing rates between the non-restraint group and repeated restraint group (right). (D) There was no significant effect of repeated restraint on the firing rate of neurons from the LAT or the BL. Box and whisker plots (left) display the median and 1.5 × IQR, the inset displays the same data at a different scale (y-axis cut at 0.8 Hz). The mean (bar) and medians (line through bar) are displayed for comparison (right). (E) Repeated restraint rats displayed significantly higher number of spontaneously firing neurons encountered per electrode track compared to non-restraint rats, indicative of increased relative number of active neurons after repeated restraint. Single restraint did not significantly impact number of spontaneously firing neurons encountered per electrode track. (F) Frequency distribution histograms of the number of spontaneously firing neurons per electrode track demonstrated different distribution between the non-restraint and repeated restraint groups. * indicates p<0.05 versus non-restraint group.
When the BLA was subdivided into LAT and BL nuclei, there was still no significant effect of repeated restraint on the firing rate in adolescent rat. Thus, there was no significant difference in the firing rate in the LAT (Fig. 4D; adolescent non-restraint: 0.13 ± 0.04 Hz, n=21 neurons; repeated restraint 0.58 ± 0.30 Hz, n=34 neurons; p>0.05, U=315, Mann-Whitney U test) or BL (Fig. 4D; adolescent non-restraint: 0.20 ± 0.05 Hz, n=19 neurons; repeated restraint: 0.41 ± 0.14 Hz, n=23 neurons; p>0.05, U=208, Mann-Whitney U test) between non-restraint and repeated restraint groups. Faster firing neurons (>0.5 Hz) were distributed approximately evenly between the LAT and BL under non-restraint (1 neuron in the LAT with a firing rate >0.5 Hz and 2 neurons in the BL with a firing rate >0.5 Hz) and repeated restraint conditions (4 neurons in the LAT with a firing rate >0.5 Hz and 5 neurons in BL with a firing rate >0.5 Hz). Removal of these neurons still did not yield a significant effect of repeated restraint stress on firing rate in the LAT (non-restraint: 0.10 ± 0.03 Hz, n=20 neurons; repeated restraint 0.09 ± 0.02 Hz, n=30 neurons; p>0.05, U=285, Mann-Whitney U test) or BL (non-restraint: 0.14 ± 0.03 Hz, n=17 neurons; repeated restraint: 0.14 ± 0.03 Hz, n=18 neurons; p>0.05, U=136.5, Mann-Whitney U test).
Adolescent rats: Effect of repeated restraint on number of active BLA neurons
To sample the relative number of active neurons, the electrode was lowered through the BLA at predefined coordinates that were the same across groups. Repeated restraint adolescent rats displayed a greater number of spontaneously firing neurons throughout the BLA compared to non-restraint rats and 1-day restraint rats, indicating a gross increase in the total number of active neurons (Fig. 4E; adolescent non-restraint: 0.47 ± 0.08 neurons/track, n=85 tracks; 1-day restraint (B) 0.61 ± 0.09 neurons/track, n=70 tracks; 1-day restraint (F) 0.44 ± 0.08 neurons/track, n=77 tracks; repeated restraint 0.93 ± 0.15 neurons/track, n=61 tracks; p=0.0034, F(3,289)=4.66, one-way ANOVA). Post hoc analysis demonstrated a significant difference in the number of active neurons between the repeated restraint group and non-restraint group (p<0.05, q=4.58, Newman-Keuls multiple comparison test) and 1-day restraint groups (1-day restraint (B) p<0.05, q=3.03; 1-day restraint (F) p<0.05, q=4.77, Newman-Keuls multiple comparison test). The frequency distribution histogram of the number of spontaneously firing neurons per electrode track demonstrated a different distribution between non-restraint group and repeated restraint group. One or more spontaneously firing neurons was observed in less than 35% of electrode tracks in the non-restraint control rats, while in repeated restraint rats, this number was approximately 52% of electrode tracks (Fig. 4F; p=0.04, χ2(1)=4.28, Chi-square test).
Adolescent rats: Effect of a single restraint
When the three control groups were compared, there was no significant difference in the firing rate (Fig. 4B; p=0.16, H(2)=3.66, Krustal-Wallis test) or the number of spontaneously firing neurons per electrode track among them (Fig. 4E; p=0.31, F(2,229)=1.16, one-way ANOVA). Therefore, a single restraint did not significantly impact BLA neuronal activity, and the impact of repeated stress on BLA neuronal activity cannot be attributed to enduring effects of the first or last restraint.
3.3 Repeated restraint increases firing rate of BLA projection neurons in adult rats
BLA neurons in adult rats
We next examined the effects of repeated restraint on neuronal activity of BLA projection neurons in adult rats. A total of 54 adult rats were included in this study (non-restraint n=16 rats; 1-day restraint (B) n=11 rats; 1-day restraint (F) n=11 rats; repeated restraint n=16 rats). A total of 185 neurons were confirmed to be within the BLA. Using action potential half-width criteria, (Fig. 5A; range: 60.88 to 856.27 μsec), 5 neurons were excluded from analysis (non-restraint n=54 neurons; 1-day restraint (B) n=34 neurons; 1-day restraint (F) n=35 neurons; repeated restraint n=57 neurons). The firing rate of BLA neurons in non-restraint adult rats was also very low (0.24 ± 0.05 Hz, n=54, range: 0.007 to 1.74 Hz). There was no significant difference in the firing rate between neurons from the LAT and from the BL under non-restraint conditions (Fig. 5B; LAT: 0.25 ± 0.06 Hz, n=34 neurons; BL: 0.22 ± 0.08 Hz, n=20 neurons; p>0.05, U=290, Mann-Whitney U test). Therefore, they were combined for initial analysis.
Figure 5. Firing of BLA neurons in adult rats.
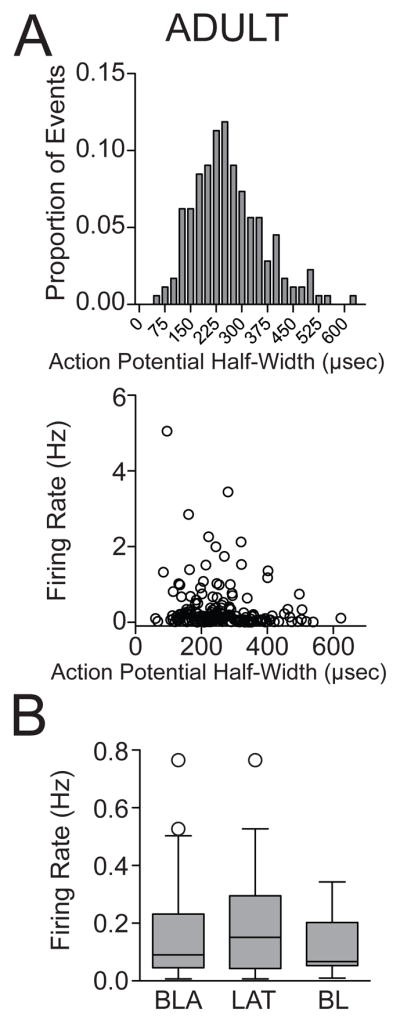
(A) Distribution histogram of action potential half-width of neurons in the BLA (top), and a plot of the action potential half-width by firing rate (bottom). (B) There was no significant difference in the firing rate between neurons from the LAT and neurons from the BL in adult rats under non-restraint conditions, displayed in this whisker plot of median and 1.5 × IQR.
Adult rats: Effect of repeated restraint on firing rate of BLA neurons
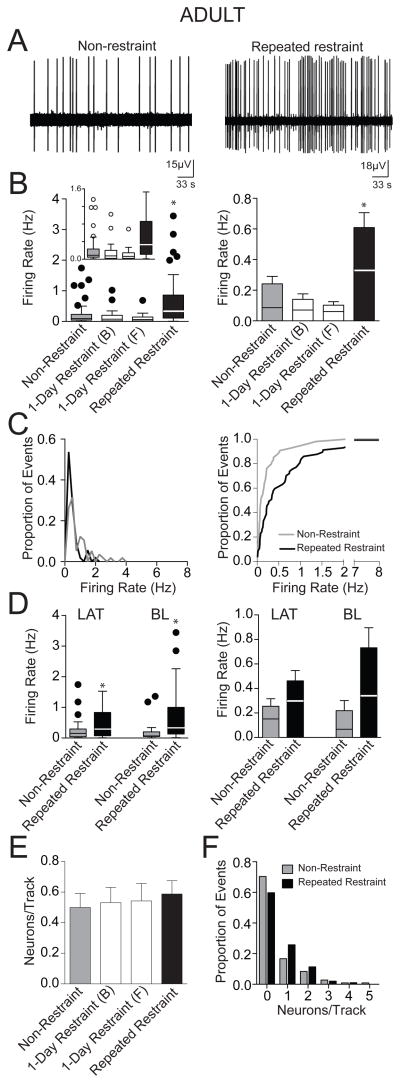
In contrast to adolescent rats, repeated restraint caused an increase in firing rate in adult rats (Fig. 6B; adult non-restraint: 0.24 ± 0.05 Hz, n=54 neurons; 1-day restraint (B) 0.14 ± 0.04 Hz, n=34 neurons; 1-day restraint (F) 0.10 ± 0.02 Hz, n=35 neurons; repeated restraint 0.61 ± 0.10 Hz, n=57 neurons; p<0.001, H(3)=32.5, Kruskal-Wallis test; post hoc significant difference between repeated restraint and non-restraint p<0.05, repeated restraint and 1-day restraint (B) and 1-day restraint (F), p<0.05, Dunn s multiple comparison test). A cumulative frequency distribution histogram of firing rate demonstrated a significantly different distribution (Fig. 6C; p<0.001, F(3,91)=11.8, comparison of parameters of best-fit to second order polynomial; p=0.001, D=0.35, K-S test). The higher firing rate after repeated restraint was observed in both LAT (Fig. 6D; adult non-restraint 0.25 ± 0.06 Hz, n=34 neurons; repeated restraint 0.46 ± 0.08 Hz, n=26 neurons; p<0.05, U=307, Mann-Whitney U test) and BL (Fig. 6D; adult non-restraint: 0.22 ± 0.08 Hz, n=20 neurons; repeated restraint: 0.73 ± 0.16 Hz, n=31 neurons; p<0.05, U=150.5, Mann-Whitney U test).
Figure 6. Repeated restraint stress increased the firing rate of BLA neurons in adult rats.
(A) A recording trace of a neuron from non-restraint rat (left) and repeated restraint rat (right). (B) Adult rats exposed to repeated restraint displayed a higher firing rate compared to non-restraint rats. Single restraint did not significantly impact firing rate. A box and whisker plot displays the median and 1.5 × IQR (left), with an inset of the same plot at a different scale (y-axis is cut-off at 1.6 Hz to facilitate comparison of the distribution and median in this inset). The overall mean (bar) and medians (line through bar) are displayed to facilitate comparison (right). (C) The distribution of firing rates in non-restraint group and repeated restraint group indicates substantial difference (left). Cumulative frequency histograms demonstrated different distributions of firing rates between the non-restraint and repeated restraint groups (right). (D) The higher firing rate after repeated restraint was observed in both neurons of the LAT and the BL of adult rats. Box and whisker plots (left) display the median and 1.5 × IQR. The mean (bar) and medians (line through bar) are displayed for comparison (right). (E) There was no significant difference in the number of spontaneously firing neurons encountered per electrode track between non-restraint and repeated restraint rats. Similarly, single restraint did not significantly impact the number of spontaneously firing neurons encountered per electrode track. (F) Frequency distribution histogram of the number of spontaneously firing neurons per electrode track demonstrated similar distribution between the non-restraint and repeated restraint groups. * indicates p<0.05
Adult rats: Effect of repeated restraint on number of active BLA neurons
There was no significant difference in the number of spontaneously firing neurons encountered per electrode track among 4 treatment groups (Fig. 6E; adult non-restraint: 0.50 ± 0.09 neurons, n=108; 1-day restraint (B) 0.53 ± 0.10 neurons/track, n=64 tracks; 1-day restraint (F) 0.55 ± 0.11 neurons/track, n=64 tracks tracks; repeated restraint: 0.59 ± 0.09 neurons, n=97 tracks; p=0.91, F(3,329)=0.17, one-way ANOVA), nor a difference in their distribution (Fig. 6F; χ2(1)=3, p=0.08, Chi-squared test).
Adult rats: Effect of a single restraint
When the three adult control groups were compared, there was no significant difference in the firing rate (Fig. 6B; p>0.05, H(2)=5, Krustal-Wallis test) or the number of spontaneously firing neurons per electrode track among them (Fig. 6E; p=0.94, F(2,233)=0.06, one-way ANOVA). Therefore, a single restraint did not significantly impact BLA neuronal activity in adult rats, and it is therefore unlikely that the effect of repeated restraint on adult BLA neuronal firing rate is caused by enduring effects of the first or last restraint session.
3.4 Adolescent and adult rats display similar neuronal activity under non-restraint conditions
Our results indicate that repeated restraint increased BLA neuronal activity in an age-dependent manner, with an increased total number of spontaneously active neurons in adolescent rats and an increased firing rate in adult rats. These differences may be due to age-dependent effects of repeated restraint stress on amygdala neuronal activity. However, to test whether differences in baseline activity contribute to the age-dependent effects, we compared BLA neuronal activity between adolescent and adult non-restraint control rats. We found that there was no significant difference in the firing rate (Fig. 7A; adolescent non-restraint 0.16 ± 0.03 Hz, n=40 neurons; adult non-restraint 0.24 ± 0.05 Hz, n=54 neurons; p>0.05, U=931.5, Mann-Whitney U test) or the number of spontaneously active neurons (Fig. 7B; adolescent non-restraint 0.47 ± 0.08 neurons/track, n=85 tracks; adult non-restraint: 0.50 ± 0.09 neurons/track, n=108 tracks; p>0,05, U=4420, Mann-Whitney U test) between these two age groups under non-restraint conditions. In addition, there was no significant difference in the firing rate in neurons from the LAT (Fig. 7A; adolescent LAT 0.13 ± 0.04 Hz, n=21 neurons; adult LAT 0.25 ± 0.06, n=34 neurons; p>0.05, U=246.5, Mann-Whitney U test) or the BL (Fig. 7A; adolescent BL 0.20 ± 0.05 Hz, n=19 neurons; adult BL 0.22 ± 0.08 Hz, n=20 neurons; p>0.05, U=171.5, Mann-Whitney U test) between adolescent and adult rats under non-restraint conditions, indicating similar neuronal activity under control conditions.
Figure 7. Adolescent and adult rats display similar neuronal activity under non-restraint conditions.
(A) There was no significant difference in the firing rate between adolescent and adult rats under non-restraint conditions when BLA was examined overall (upper), nor when subdivided into LAT (middle) and BL (bottom). (B) There was no significant difference in the number of spontaneously firing neurons per electrode track between adolescent and adult rats under non-restraint conditions.
4 DISCUSSION
This study demonstrated that repeated restraint induces substantially different alterations in spontaneous activity of BLA projection neurons in adolescent and adult rats. Repeated restraint exposure increased the relative number of spontaneously active BLA neurons in adolescent rats, but not in adult rats. However, the same restraint exposure increased the firing rate of BLA neurons in adult rats, but not in adolescent rats. These findings are the first to report the effects of repeated restraint on BLA neuronal activity in adolescent rats, and age-dependent differences in how a repeated stressor influences BLA neuronal activity.
4.1 Repeated restraint protocol
Previous research has shown that early-life adverse experience such as prolonged or repeated stress induces behavioral abnormalities in adulthood (Heim and Nemeroff, 2001; Teicher et al, 2003). However, the specific physiological changes in adolescents and how this differs from the effects on adults have not been studied in detail. This is partially due to the prolonged stress protocols used in other studies (21 days or longer; Vyas, et al, 2006; Toth et al, 2008) and the relatively short duration of adolescence in rodent (approximately PND 28 – 42; Spear, 2000). The 7-day restraint protocol in this study allowed us to stress and test rats during adolescence and compare the results to adult rats exposed to the same severity and duration of stress. Restraint causes fluctuation of stress hormones such as corticosterone, which may in turn contribute to many behavioral and morphological changes observed after restraint. In this study, we measured adrenal gland weight and exploration in the EPM as independent confirmation of the effectiveness of the restraint protocol as a repeated stressor. Our restraint protocol resulted in increased anxiety-like behaviors (Fig. 1) and adrenal gland hypertrophy (Fig. 1). These behavioral and endocrine changes are consistent with findings reported by other labs using repeated stressors of different types or duration (Gomez et al, 1996; Márquez et al, 2004; Vyas et al, 2004, 2006; Pohl et al, 2007). However, due to differences in growth rates and exploratory behavior across ages, from the current study it is difficult to determine whether repeated restraint stress had a greater impact on EPM behavior or adrenal gland across ages.
Furthermore, as evidenced by the lack of effect in the two single restraint control groups, the impact of repeated restraint was due to its repeated nature. Some previous reports show that a single exposure to stressors results in anxiogenic response (e.g. Albonetti and Farabollini, 1992; Heinrichs et al, 1994; McBlane and Handley, 1994; Padovan and Guimarães, 2000; Cecchi et al, 2002; Korte and De Boer, 2003; Belda et al, 2008) (but see also Mitra et al, 2005; Muñoz-Abellán et al, 2011). However, there are important differences in those studies. In some of those studies, the EPM experiment was carried out within hours after the stress session (e.g. Albonetti and Farabollini, 1992; Heinrichs et al, 1994; Padovan and Guimarães, 2000; Cecchi et al, 2002; Korte and De Boer, 2003). In our study, all EPM experiments were performed 24 hours after the last restraint session, when the presumed acute effects of the stressor have returned to baseline. In addition, some of the studies used more severe stressors, such as footshock, longer restraint, social defeat and cold swim. But our study used a 20 min restraint stressor, which is a relatively milder stressor. Studies that utilize longer restraint tend to find prolonged effects on EPM (e.g. Guimarães et al, 1993; Padovan et al, 2000). Studies that have compared the impact of restraint duration have found that longer lasting restraint can exert qualitatively different effects in the EPM measured immediately following restraint (McBlane and Handley, 1994) or one day later (e.g. Belda et al, 2008). A different study found that a single restraint did not lead to a change in EPM measured 1 day later, but did lead to a change measured 10 days later (Mitra et al, 2005). In that study, rats were subjected to complete immobilization in a rodent bag, not in cylinders used in the current study, and were immobilized for a longer period of time. However, one study found anxiogenic effects of brief restraint (15min) on the EPM when measured one day later (Martijena et al, 1997). The same group found that these effects were absent if the rats were allowed to chew during the restraint (Martijena et al, 1997). Rats in our experiments were able to chew on the cylinder. Furthermore, in the single restraint control group in our study, rats experienced 6 days of daily handling, which may counteract some of the anxiogenic effect of the restraint, while some of the other studies appear to have used non-handled controls as the comparison group (e.g. Mitra et al, 2005).
4.2 Activity of BLA projection neurons
The BLA receives information from multisensory modalities. It plays an important role in regulation of affective behaviors and influences the hormonal, autonomic, and behavioral responses to various affective stimuli via its widespread connection to other brain regions (LeDoux 2000; Rodrigues et al, 2004). Within the BLA, the glutamatergic projection neurons are the main output neurons that largely determine the impact of the amygdala on other structures (McDonald, 1984; Pitkanen et al, 1997). Under normal conditions, BLA projection neurons are subjected to intense suppression by GABAergic inputs (Muller et al, 2006; Woodruff and Sah, 2007). Therefore, the majority of projection neurons are silent or show scant spontaneous activity, as indicated by their low firing rate (Fig. 3, 5). Similar results have been reported previously (Rosenkranz and Grace, 1999; Likhtik et al, 2006). Rats in this study were anesthetized with urethane. This provides a stable firing of BLA neurons for comparison across conditions. However, even though urethane is widely used as anesthetic in electro-physiological recording, we cannot exclude the contribution of anesthesia to the low BLA neuronal activity observed in this study. In addition, the anesthesia state may have contributed to a lack of significant effects of stress on firing rate in adolescent rats. Future experiments in freely moving animals might provide a better understanding of how a repeated stressor changes BLA neuronal activity during behavior. This technique could provide information about the activity of individual neurons and population activity (Quirk et al, 1995; Pare and Collins, 2000; Repa et al, 2001; Fontanini et al, 2009) in response to sensory stimuli.
Previous studies have used firing rate and action potential duration to differentiate projection neurons from putative interneurons (projection neurons tend to have low firing rate and longer action potential duration) (Rosenkranz and Grace, 2001; Likhtik et al 2006). Based on those studies, and the distribution of values in this study, we chose a cut-off of 100 μsec action potential half-width to eliminate potential interneurons. Action potential duration is partially determined by the distance of the electrode from the recorded neuron, which is difficult to control in in vivo studies. However, measurement of half-width is expected to be less sensitive to distance of the electrode from the neuron, compared to full biphasic action potential duration. In addition, as interneurons make up a smaller population of BLA neurons (McDonald, 1992; McDonald and Augustine, 1993), we believed that our results primarily represent the firing activity of projection neurons.
In addition, several studies have reported that neurons in the LAT and BL have subtle differences in morphological and physiological properties (Millhouse and DeOlmos, 1983; McDonald, 1992; Paré et al, 1995; Paré and Gaudreau, 1996), and exert different functions, especially in Pavlovian fear conditioning (Amano et al, 2011). In our study, we did not observe any significant difference in the firing rate between neurons from LAT and neurons from BL under non-restraint conditions in both adolescent and adult rats (Fig. 3, 5). Repeated restraint resulted in an increase in the firing rate in adult rats. However, this increase occurred in both LAT and BL (Fig. 6).
4.3 Effect of repeated restraint on BLA neuronal activity in adolescent and adult rats
Numerous studies have shown changes in amygdala structure and function over development, and that exposure to stress disrupts the normal development of the amygdala. The volume of amygdala, especially the BLA in rats, increases by 113% from birth to 3 week old with an additional 33% increased by 7 month of age (Chareyron et al 2012). However, there is evidence of neurogenesis in the amygdala across ages (Kordower et al, 1992; Bernier et al, 2002; Fudge, 2004). Corresponding to development and adolescence, there are changes in amygdala function and anatomy in humans (Giedd et al, 1996), and adolescent humans tend to display greater amygdala activation to fearful faces than adults (Monk et al, 2003; Guyer et al, 2008). Furthermore, effects of amygdala lesions are dependent upon the age at the lesion in non-human primates (Prather et al, 2001; Bauman et al, 2004). In fact, the role of the BLA in aversive learning may not develop until more than 10 days after birth in rats (Roth and Sullivan, 2005; Shionoya et al, 2006; Raineki et al, 2009). Amygdala-dependent fear conditioning also varies across later development, as demonstrated by differences in expression modes, degree of expression, and acquisition (McKinzie et al, 1998; Richardson et al, 2000; Stanton, 2000; Richarson and Fan, 2002; Barnet and Hunt, 2005; Hefner and Holmes, 2007; Yap and Richardson, 2007). Stress exposure during this later period of development reshapes the structure and function of amygdala. For example, stressor exposure leads to the changes in expression of certain GABA(A) receptor subunits in amygdala (Jacobson-Pick et al, 2008), reduces apical spine densities in medial part of lateral amygdala (Poeggel et al 2003), reduces amygdala 5-HT innervation (Kuramochi and Nakamura, 2009), decreases CB1 receptor expression (Malone et al, 2008) and increases DA D2 receptors in the amygdala (Djouma et al, 2006), and modifies molecules involved in neural circuit development (Leussis and Andersen, 2008; Tsoory et al, 2008, 2010; Gilabert-Juan et al, 2012) among the wide range of changes. It also leads to long-lasting changes in affective behaviors (e.g. Maslova et al, 2002; Avital and Richter-Levin, 2005; Tsoory and Richter-Levin, 2006; Vidal et al, 2007; Lukkes et al, 2009; Saul et al, 2012). In addition, changes induced by early-life adverse experience also occurred in other brain regions involved in the stress response circuits, such as hippocampus and medial prefrontal cortex (Sunanda et al, 1995; Silva-Gomez et al, 2003; Lippmann et al, 2007; Leussis and Andersen, 2008). In conjunction with greater and longer lasting neuroendocrine responses to stress exposure in adolescent rats (Sapolsky and Meaney, 1986; Walker et al, 1991; Romeo et al, 2006), adolescence may be a time period of differential sensitivity to stress and stress-induced abnormal affective behaviors.
The results from this study demonstrate that repeated restraint induces BLA hyperactivity in both adolescent and adult rats, but this manifests in a different manner in these age groups. Although BLA neuronal activity is similar under non-restraint conditions between these two age groups (Fig. 7), adolescent rats exposed to repeated restraint exhibited increased relative number of active neurons in the BLA, indicating a gross increase in the population activity. Compared to adult rats, this enhanced population activity may translate to greater influence on amygdala-dependent behaviors by recruitment of more neurons and therefore greater output from the BLA in adolescent rats. The specific changes in neuronal activity after repeated stress may result in different stress-induced changes in amygdala-dependent affective behaviors. Fear conditioning relies strongly upon the BLA. Other studies indicate that repeated restraint exerts distinct effects on BLA-mediated conditioned freezing and extinction in adult and adolescent rats (Toledo-Rodriguez and Sandi, 2007; Zhang et al, 2010). Although the increased BLA neuronal activity may contribute to changes in fear conditioning, other factors such as changes in the interaction between mPFC and amygdala cannot be neglected. In addition, the increased number of spontaneously active neurons and increased basal firing rate in adolescent and adult respectively may suggest different underlying mechanisms of structural and molecular changes of BLA after stress exposure. Therefore, early age onset affective disorders and adult onset disorders may require different treatments as well as different diagnosis criteria. Moreover, while several studies indicate that early life stress contribute to life-long physiological and behavioral abnormalities (Kaufman and Charney, 1999; Heim and Nemeroff, 2001; Danese et al 2007), our current study in adolescent rats did not address whether repeated stressor has long lasting effect or whether early life stress contributes to a greater vulnerability of affective disorders in adulthood. Therefore, future research using adult animals exposed to adolescent stress would provide important information.
4.4 Underlying mechanisms of increased neuronal activity after repeated restraint
Many factors may contribute to the increased activity of BLA projection neurons observed after repeated restraint or other chronic stressors in both adolescent and adult rats. First, chronic stress results in the alteration of neurotransmitters or their receptors, such as dopamine (Rasheed et al, 2010) and neuropeptide Y (Thorsell et al, 2006), which may lead to abnormalities in their function in regulation of neuronal activity. A second mechanism by which neuronal activity is altered after repeated stress is via the decrease of inhibition (Caldji et al 2003; Braga et al, 2004). In addition, the abnormal function of ion channels, such as calcium-activated potassium channels, which results in the hyper-excitability and hyper-responsiveness of BLA projection neurons may also play a role in the alterations of neuronal activity after repeated stress (Rosenkranz et al 2010). Moreover, stress-induced BLA neuronal hypertrophy, including increased number of spines and elongation of dendrites, may reflect enhanced excitatory drive (Vyas et al, 2004, 2006). This in turn will contribute to changes in BLA neuronal activity. However, it is still not known which of these, or other, potential mechanisms lead to a change in firing rate versus a change in the number of active BLA neurons. It is expected that targeting of these respective changes can lead to age-specific reversal of the effects of repeated stress on amygdala-mediated affective behaviors.
In addition, corticotropin-releasing factor (CRF) directly acts on several brain structures involved in stress, and mediates several aspects of the effects of stress on amygdala function. The amygdala, including the BLA, has been shown to express high level of CRF receptors as well as their mRNA (Chalmers et al, 1995; Chen, et al 2000). Stress leads to the release of CRF in the BLA (Merlo et al, 1995). In vitro research has shown that CRF receptor activation increases the excitability of BLA projection neurons (Rainnie et al, 1992), and repeated activation of CRF receptors in the BLA results in anxiety-like responses and reduction of inhibition within the BLA (Rainnie et al, 2004). This evidence suggests that CRF may contribute to the chronic stress-induced hyperactivity in the amygdala. Furthermore, CRF mRNA levels in the BLA are age-dependent (Eghbal-Ahmadi et al, 1998; Vazquez et al 2006), and there is an age-dependency in the ability of stressors to cause changes in CRF receptor expression in the amygdala (Kalin et al, 1994; Hatalski et al, 1998; Vazquez et al, 2006). These factors may contribute to the age-dependent effects of stress on amygdala neuronal activity observed here.
4.5 Implications
BLA neuronal activity is tightly regulated. This assures that the amygdala only responds to emotionally salient stimuli, and triggers accurate and rapid affective responses. However, following prolonged or repeated stress, abnormally high neuronal activity in conjunction with hyper-excitability and hyper-responsiveness of projection neurons may lead to inappropriate responses to normally sub-threshold stimuli. Changes in different aspects of neuronal activity between adolescent and adult rats suggest different underlying mechanisms of stress-induced abnormal affective behaviors between these two age groups. These findings may help explain the age-dependent impact of chronic stress on amygdala-dependent behaviors and affective disorders and contribute to the development of age-appropriate treatment for certain affective disorders.
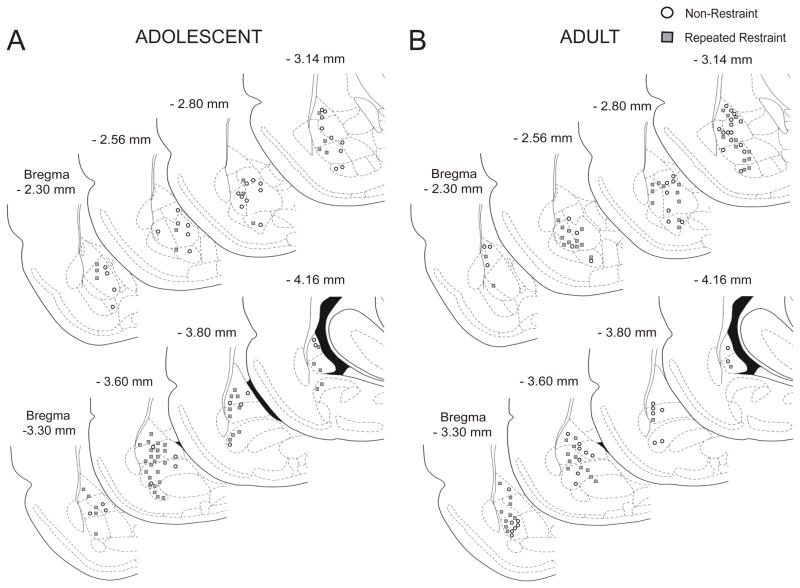
Figure 2. Location of neurons recorded in the BLA.
Neurons were histologically verified to lie within the BLA, based on the reconstruction of their location from the Pontamine Sky Blue marker made at the end of each electrophysiological experiment. Neurons recorded from non-restraint rats were marked with open circle. Neurons recorded from repeated restraint rats were marked with grey square. (A) Location of neurons recorded in the BLA in adolescent rats. (B) Location of neurons recorded in the BLA in adult rats.
HIGHLIGHTS.
Repeated restraint increased BLA neuronal activity in an age-dependent manner.
Repeated restraint increased the number of active BLA neurons in adolescent rats.
Repeated restraint increased the firing rate of BLA neurons in adult rats.
Acknowledgments
The author wishes to thank Mallika Padival for help with histological processing of brain tissue, and Drs. Anthony West, Beth Stutzmann, Janice Urban and Michela Marinelli for useful discussion.
GRAND
Grant support provided by the U.S. National Institutes of Health (MH084970) and the Brain Research Foundation.
ABBREVIATIONS
- ANOVA
Analysis of Variance
- BL
basal nucleus
- BLA
basolateral amygdala
- CRF
corticotropin-releasing factor
- EEG
electroencephalogram
- EPM
elevated plus maze
- HPA axis
hypothalamic–pituitary–adrenal axis
- K-S
Kolmogorov-Smirnov
- LAT
lateral nucleus
- mPFC
medial prefrontal cortex
- PND
postnatal day
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose. W.Z. and J.A.R conceived and designed experiments, W.Z. collected and analyzed data, W.Z. and J.A.R. interpreted data, drafted and revised article. Both authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALBONETTI ME, FARABOLLINI F. Behavioral responses to single and repeated restraint in male and female rats. Behavioral Processes. 1992;28:97–109. doi: 10.1016/0376-6357(92)90052-F. [DOI] [PubMed] [Google Scholar]
- AMANO T, DUVARCI S, POPA D, PARE D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVITAL A, RICHTER-LEVIN G. Exposure to juvenile stress exacerbates the behavioral consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- BARNET RC, HUNT PS. Trace and long-delay fear conditioning in the developing rat. Learn Behav. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- BAUMAN MD, LAVENEX P, MASON WA, CAPITANIO JP, AMARAL DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- BELDA X, FUENTES S, NADAL R, ARMARIO A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- BERNIER PJ, BEDARD A, VINET J, LEVESQUE M, PARENT A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGA MF, ARONIADOU-ANDERJASKA V, MANION ST, HOUGH CJ, LI H. Stress impairs alpha (1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- BREMNER JD, ELZINGA B, SCHMAHL C, VERMETTEN E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUFFALARI DM, GRACE AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDJI C, DIORIO J, MEANEY MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- CECCHI M, KHOSHBOUEI H, MORILAK DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- CHALMERS DT, LOVENBERG TW, DE SOUZA EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAREYRON LJ, LAVENEX PB, LAVENEX P. Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol. 2012 doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- CHEN Y, BRUNSON KL, MULLER MB, CARIAGA W, BARAM TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONRAD CD, LEDOUX JE, MAGARINOS AM, MCEWEN BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- CORRELL CM, ROSENKRANZ JA, GRACE AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- DANESE A, PARIANTE CM, CASPI A, TAYLOR A, POULTON R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M, RAINNIE D, CASSELL M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- DJOUMA E, CARD K, LODGE DJ, LAWRENCE AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci. 2006;23:3319–3327. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- DREVETS WC, VIDEEN TO, PRICE JL, PRESKORN SH, CARMICHAEL ST, RAICHLE ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGHBAL-AHMADI M, HATALSKI CG, LOVENBERG TW, AVISHAI-ELINER S, CHALMERS DT, BARAM TZ. The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res Dev Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN S, CONFORTI N, WEIDENFELD J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci Biobehav Rev. 1995;19:235–240. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- FONTANINI A, GROSSMAN SE, FIGUEROA JA, KATZ DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J Neurosci. 2009;29(8):2486–2495. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRODL T, MEISENZAHL E, ZETZSCHE T, BOTTLENDER R, BORN C, GROLL C, JAGER M, LEINSINGER G, HAHN K, MOLLER HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- FUDGE JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEDD JN, VAITUZIS AC, HAMBURGER SD, LANGE N, RAJAPAKSE JC, KAYSEN D, VAUSS YC, RAPOPORT JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- GILABERT-JUAN J, MOLTO MD, NACHER J. Post-weaning social isolation rearing influences the expression of molecules related to inhibitory neurotransmission and structural plasticity in the amygdala of adult rats. Brain Res. 2012;1448:129–136. doi: 10.1016/j.brainres.2012.01.073. [DOI] [PubMed] [Google Scholar]
- GOMEZ F, LAHMAME A, DE KLOET ER, ARMARIO A. Hypo- thalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- GUIMARAES FS, DEL BEL EA, PADOVAN CM, NETTO SM, DE ALMEIDA RT. Hippocampal 5-HT receptors and consolidation of stressful memories. Behav Brain Res. 1993;58:133–139. doi: 10.1016/0166-4328(93)90098-b. [DOI] [PubMed] [Google Scholar]
- GUYER AE, MONK CS, MCCLURE-TONE EB, NELSON EE, ROBERSON-NAY R, ADLER AD, FROMM SJ, LEIBENLUFT E, PINE DS, ERNST M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMEN C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- HATALSKI CG, GUIRGUIS C, BARAM TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEFNER K, HOLMES A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIM C, NEMEROFF CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- HEINRICHS SC, MENZAGHI F, PICH EM, BALDWIN HA, RASSNICK S, BRITTON KT, KOOB GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 1994;11:179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- HERMAN JP, CULLINAN WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- ISOARDI NA, BERTOTTO ME, MARTIJENA ID, MOLINA VA, CARRER HF. Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci. 2007;26:1036–1044. doi: 10.1111/j.1460-9568.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- JACOBSON-PICK S, ELKOBI A, VANDER S, ROSENBLUM K, RICHTER-LEVIN G. Juvenile stress-induced alteration of maturation of the GABAA receptor alpha subunit in the rat. Int J Neuropsychopharmacol. 2008;11:891–903. doi: 10.1017/S1461145708008559. [DOI] [PubMed] [Google Scholar]
- JIANG X, XING G, YANG C, VERMA A, ZHANG L, LI H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- KALIN NH, TAKAHASHI LK, CHEN FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- KARST H, BERGER S, ERDMANN G, SCHUTZ G, JOELS M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN J, CHARNEY DS. Neurobiological correlates of child abuse. Biol Psychiatry. 1999;45(10):1235–1236. doi: 10.1016/s0006-3223(99)00064-5. [DOI] [PubMed] [Google Scholar]
- KAVUSHANSKY A, RICHTER-LEVIN G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res. 2006;84:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, AVENEVOLI S, RIES MERIKANGAS K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- KORDOWER JH, PIECINSKI P, RAKIC P. Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- KORTE SM, DE BOER SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- KURAMOCHI M, NAKAMURA S. Effects of postnatal isolation rearing and antidepressant treatment on the density of serotonergic and noradrenergic axons and depressive behavior in rats. Neuroscience. 2009;163:448–455. doi: 10.1016/j.neuroscience.2009.06.017. [DOI] [PubMed] [Google Scholar]
- LEDOUX JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LEUSSIS MP, ANDERSEN SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- LIKHTIK E, PELLETIER JG, POPESCU AT, PARE D. Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J Neurophysiol. 2006;96:3257–3265. doi: 10.1152/jn.00577.2006. [DOI] [PubMed] [Google Scholar]
- LIPPMANN M, BRESS A, NEMEROFF CB, PLOTSKY PM, MONTEGGIA LM. Long-term behavioral and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- LUKKES JL, MOKIN MV, SCHOLL JL, FORSTER GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- MALONE DT, KEARN CS, CHONGUE L, MACKIE K, TAYLOR DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152:265–272. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARQUEZ C, NADAL R, ARMARIO A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–612. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- MARTIJENA ID, CALVO N, VOLOSIN M, MOLINA VA. Prior exposure to a brief restraint session facilitates the occurrence of fear in response to a conflict situation: behavioral and neurochemical correlates. Brain Res. 1997;752:136–142. doi: 10.1016/s0006-8993(96)01465-5. [DOI] [PubMed] [Google Scholar]
- MASLOVA LN, BULYGINA VV, MARKEL AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–561. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- MCBLANE JW, HANDLEY SL. Effects of two stressors on behaviour in the elevated X-maze: preliminary investigation of their interaction with 8-OH-DPAT. Psychopharmacology (Berl) 1994;116:173–182. doi: 10.1007/BF02245060. [DOI] [PubMed] [Google Scholar]
- MCDONALD AJ. Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. J Comp Neurol. 1984;222:589–606. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- MCDONALD AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- MCDONALD AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- MCDONALD AJ, AUGUSTINE JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- MCKINZIE DL, CHEN WJ, SPEAR NE. Ontogenetic differences in the expression of conditioned stimulus conditioning: effects of retention interval. Behav Neurosci. 1998;112:920–928. doi: 10.1037//0735-7044.112.4.920. [DOI] [PubMed] [Google Scholar]
- MERIKANGAS KR, HE JP, BURSTEIN M, SWANSON SA, AVENEVOLI S, CUI L, BENJET C, GEORGIADES K, SWENDSEN J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERLO PICH E, LORANG M, YEGANEH M, RODRIGUEZ DE FONSECA F, RABER J, KOOB GF, WEISS F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLHOUSE OE, DEOLMOS J. Neuronal configurations in lateral and basolateral amygdala. Neuroscience. 1983;10:1269–1300. doi: 10.1016/0306-4522(83)90112-4. [DOI] [PubMed] [Google Scholar]
- MITRA R, JADHAV S, MCEWEN BS, VYAS A, CHATTARJI S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONK CS, MCCLURE EB, NELSON EE, ZARAHN E, BILDER RM, LEIBENLUFT E, CHARNEY DS, ERNST M, PINE DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- MOZHUI K, KARLSSON RM, KASH TL, IHNE J, NORCROSS M, PATEL S, FARRELL MR, HILL EE, GRAYBEAL C, MARTIN KP, CAMP M, FITZGERALD PJ, CIOBANU DC, SPRENGEL R, MISHINA M, WELLMAN CL, WINDER DG, WILLIAMS RW, HOLMES A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER JF, MASCAGNI F, MCDONALD AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNOZ-ABELLAN C, RABASA C, DAVIU N, NADAL R, ARMARIO A. Behavioral and endocrine consequences of simultaneous exposure to two different stressors in rats: interaction or independence? PLoS One. 2011;6:e21426. doi: 10.1371/journal.pone.0021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADOVAN CM, DEL BEL EA, GUIMARAES FS. Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: influence of restraint stress. Pharmacol Biochem Behav. 2000;67:325–330. doi: 10.1016/s0091-3057(00)00361-0. [DOI] [PubMed] [Google Scholar]
- PADOVAN CM, GUIMARAES FS. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33:79–83. doi: 10.1590/s0100-879x2000000100011. [DOI] [PubMed] [Google Scholar]
- PARE D, COLLINS DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20(7):2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARE D, GAUDREAU H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARE D, PAPE HC, DONG J. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol. 1995;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- PATEL S, KINGSLEY PJ, MACKIE K, MARNETT LJ, WINDER DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLETIER JG, LIKHTIK E, FILALI M, PARE D. Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAXINOS G, WATSON C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- PITKANEN A, SAVANDER V, LEDOUX JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- POEGGEL G, HELMEKE C, ABRAHAM A, SCHWABE T, FRIEDRICH P, BRAUN K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci USA. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POHL J, OLMSTEAD MC, WYNNE-EDWARDS KE, HARKNESS K, MENARD JL. Repeated exposure to stress across the childhood-adolescent period alters rats anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- PRATHER MD, LAVENEX P, MAULDIN-JOURDAIN ML, MASON WA, CAPITANIO JP, MENDOZA SP, AMARAL DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- PROTOPOPESCU X, PAN H, TUESCHER O, CLOITRE M, GOLDSTEIN M, ENGELIEN W, EPSTEIN J, YANG Y, GORMAN J, LEDOUX J, SILBERSWEIG D, STERN E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- QUIRK GJ, REPA JC, LEDOUX JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15(5):1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- RAINEKI C, SHIONOYA K, SANDER K, SULLIVAN RM. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINNIE DG, BERGERON R, SAJDYK TJ, PATIL M, GEHLERT DR, SHEKHAR A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINNIE DG, FERNHOUT BJ, SHINNICK-GALLAGHER P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- RASHEED N, AHMAD A, PANDEY CP, CHATURVEDI RK, LOHANI M, PALIT G. Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem Res. 2010;35:22–32. doi: 10.1007/s11064-009-0026-5. [DOI] [PubMed] [Google Scholar]
- RAUCH SL, WHALEN PJ, SHIN LM, MCINERNEY SC, MACKLIN ML, LASKO NB, ORR SP, PITMAN RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- REPA JC, MULLER J, APERGIS J, DESROCHERS TM, ZHOU Y, LEDOUX JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4(7):724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- RICHARDSON R, FAN M. Behavioral expression of learned fear in rats is appropriate to their age at training, not their age at testing. Anim Learn Behav. 2002;30:394–404. doi: 10.3758/bf03195964. [DOI] [PubMed] [Google Scholar]
- RICHARDSON R, PAXINOS G, LEE J. The ontogeny of conditioned odor potentiation of startle. Behav Neurosci. 2000;114:1167–1173. [PubMed] [Google Scholar]
- RODRIGUES SM, SCHAFE GE, LEDOUX JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- ROMEO RD, KARATSOREOS IN, MCEWEN BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- ROTH TL, SULLIVAN RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ JA, GRACE AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENKRANZ JA, GRACE AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENKRANZ JA, VENHEIM ER, PADIVAL M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAPOLSKY RM, MEANEY MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- SAUL ML, TYLEE D, BECOATS KT, GUERRERO BG, SWEENEY P, HELMREICH DL, FUDGE JL. Long-term behavioral consequences of stress exposure in adolescent versus young adult rats. Behav Brain Res. 2012;229:226–234. doi: 10.1016/j.bbr.2012.01.022. [DOI] [PubMed] [Google Scholar]
- SHIONOYA K, MORICEAU S, LUNDAY L, MINER C, ROTH TL, SULLIVAN RM. Development switch in neural circuitry underlying odor-malaise learning. Learn Mem. 2006;13:801–808. doi: 10.1101/lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORS TJ. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport. 1999;10:2811–2815. doi: 10.1097/00001756-199909090-00021. [DOI] [PubMed] [Google Scholar]
- SHORS TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA-GOMEZ AB, ROJAS D, JUAREZ I, FLORES G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- SISK CL, FOSTER DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- SPEAR LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- STANTON ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- STONE EA, QUARTERMAIN D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- SUNANDA, RAO MS, RAJU TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons--a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, ANDERSEN SL, POLCARI A, ANDERSON CM, NAVALTA CP, KIM DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, TOMODA A, ANDERSEN SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- THORSELL A, SLAWECKI CJ, EL KHOURY A, MATHE AA, EHLERS CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- TOLEDO-RODRIGUEZ M, SANDI C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOTH E, GERSNER R, WILF-YARKONI A, RAIZEL H, DAR DE, RICHTER-LEVIN G, LEVIT O, ZANGEN A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- TSOORY M, GUTERMAN A, RICHTER-LEVIN G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- TSOORY M, GUTERMAN A, RICHTER-LEVIN G. “Juvenile stress” alters maturation-related changes in expression of the neural cell adhesion molecule L1 in the limbic system: relevance for stress-related psychopathologies. J Neurosci Res. 2010;88:369–380. doi: 10.1002/jnr.22203. [DOI] [PubMed] [Google Scholar]
- TSOORY M, RICHTER-LEVIN G. Learning under stress in the adult rat is differentially affected by juvenile or adolescent stress. Int J Neuropsychopharmacology. 2006;9:713–728. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- TURNER BH, HERKENHAM M. Thalamoamygdaloid projections in the rat: a test of the amygdala s role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- VAN DE KAR LD, BLAIR ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- VAZQUEZ DM, BAILEY C, DENT GW, OKIMOTO DK, STEFFEK A, LOPEZ JF, LEVINE S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIDAL J, BIE J, GRANNEMAN RA, WALLINGA AE, KOOLHAAS JM, BUWALDA B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- VOUIMBA RM, MUNOZ C, DIAMOND DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- VOUIMBA RM, YANIV D, DIAMOND D, RICHTER-LEVIN G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur J Neurosci. 2004;19:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- VYAS A, CHATTARJI S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- VYAS A, JADHAV S, CHATTARJI S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- VYAS A, MITRA R, SHANKARANARAYANA RAO BS, CHATTARJI S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYAS A, PILLAI AG, CHATTARJI S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- WALKER CD, SCRIBNER KA, CASCIO CS, DALLMAN MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- WOOD GE, NORRIS EH, WATERS E, STOLDT JT, MCEWEN BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- WOODRUFF AR, SAH P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAP CS, RICHARDSON R. The ontogeny of fear-potentiated startle: effects of earlier-acquired fear memories. Behav Neurosci. 2007;121:1053–1062. doi: 10.1037/0735-7044.121.5.1053. [DOI] [PubMed] [Google Scholar]
- ZHANG W, PADIVAL M, ROSENKRANZ JA. Chronic stress increases neuronal activity in the basolateral amygdala of adolescent rats. Soc Neurosci. 2010:Abstr. 905.5. [Google Scholar]