The majority of tuberculosis (TB) cases are due to pulmonary tuberculosis (PTB). The main management approach is to eliminate the pathogen at its source. Thus, an understanding and good knowledge of PTB will be essential for controlling the spread and dissemination of TB. This article summarizes the up-to-date research advances in chemotherapy, interventional therapy, surgical treatment, immunotherapy, and TCM therapy for PTB.
Chemotherapy
As a dominant treatment for PTB, chemotherapy, with the use of antimicrobial drugs, can exert its antibacterial effect primarily through interfering with the biochemical metabolism of Mycobacterium tuberculosis (MTB) and affecting its structure and function of bacteria. In addition to the traditional first-line anti-TB drugs such as isoniazid, ethambutol, rifampicin, pyrazinamide, and streptomycin, the current targets for anti-TB drugs under development include the key enzymes in the glyoxylate cycle and biosynthesis of amino acids, the key enzymes in biosynthesis of nucleic acids, the key enzymes and carriers in macromolecular biosynthesis of the cell wall as well as biosynthesis of menaquinones and pantothenic acids (1). The new drugs are expected to inhibit bacterial cell wall synthesis, suppress cell membrane function, inhibit or interfere with protein synthesis, and affect nucleic acid metabolism.
Inhibiting cell wall synthesis
The cellular wall of MTB consists of three kinds of structure covalently attached: peptidoglycan, arabinogalactan, and mycolic acid. The peptidoglycan layer is a key component, with statically cross-linking structure linked mainly by 4→3 transpeptidase. Nonetheless, a nonclassical 3→3 linking mode dominates the network structure of peptidoglycan in non-replicated MTB. According to Gupta R. et al. (2), MT2594 (Rv2518c) dominates the growth phase of MTB through affecting the 3→3 linking mode as a kind of L,D-transpeptidase; such protein loss will lead to a change in colony morphology and loss of virulence while increasing the susceptibility of the host to amoxicillin-clavulanate potassium during chronic infection. Thus, L,D-transpeptidase and β-lactamase inhibitor complexes, such as amoxicillin-clavulanate potassium, can effectively inhibit the growth of MTB. Cycloserine can inhibit peptidoglycan synthesis through suppressing alanine racemase and its synthetases; by doing so, it can cause MTB cell wall defects, attenuate its acid tolerance, and thus exert its bactericidal and bacteriostatic effects (3). Isoniazid can achieve its anti-TB purpose through inhibiting MTB mycolic acid synthesis by suppressing the Inh A enzyme.
Currently, the potential drug targets in arabinogalactan synthesis include UbiA, glycosyl transferase, regulatory factors, RmlA, RmlB, and RmlC (4-8). Wherein RmlA, RmlB and RmlC rmlA (Rv0334) encode D-glucose-1-thymine ribonucleoside phosphate transferase, rmlB encode dTDP-D-glucose-4,6-dehydratase, rmlC (Rv3465) encode dTDP-4-keto-6-deoxyglucose epimerase and thus are involved in the formation of p-GlcNAc-Rha linking units between AG and peptidoglycan and play important roles in MTB cell wall construction (9,10). Aderwick et al. found in 2005 that arabinofuranosyltransferase (AftA) is not only a key enzyme in the biosynthetic pathway of arabinan, one of the main components of the cell wall of MTB, but also an important MTB virulence-associated factor; meanwhile, as a novel AftA, AftB and AftC respectively catalyzes the formation of the β and α bond between Araf, i.e., the formation of the terminal AG sites for mycolic acid attachment, with the encoding genes of Rv3805 and Rv2673 (11-14), which provide novel targets of anti-TB drugs.
Interfering with the function of the cytoplasmic membrane
Azole antifungal agents have anti-TB effects; particularly, econazole has inhibitory effects on MTB with vigorous metabolism, MTB at the resting phase, multidrug-resistant MTB, and murine MTB (15-20). Lee et al. observed the anti-TB effects of nitroimidazole compounds via econazole, in particular, when there is no difference in the activity of 6f (i.e., Figure 1, R1 is 4-phenyl) against MTB at the resting phase when compared with PA-824 (21). Till now, two crystal structures of the P450 enzyme of MTB have already been identified: CYP5l and CYPl21. Despite the undefined physiological role, CYPl21 may still be a real target of azoles due to its high affinity to azoles, which may provide a new platform for the design of novel anti-TB drugs.
Figure 1.
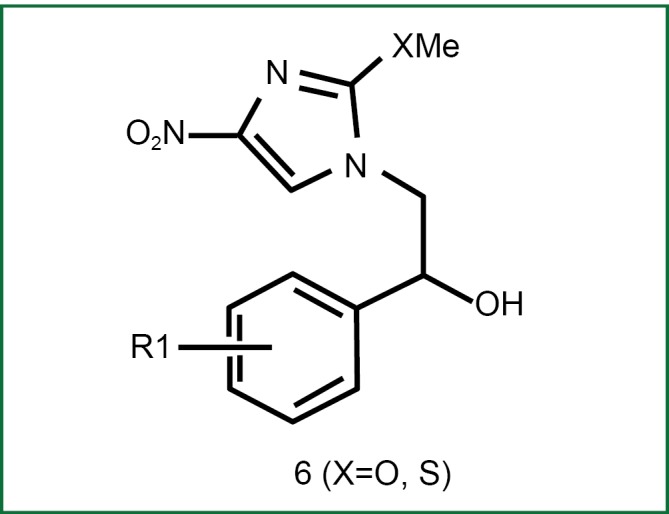
Alcoholic nitroimidazoles.
Inhibiting or interfering with protein synthesis
Aminoglycosides
By acting on the ribosome of MTB, aminoglycosides induce the misreading of genetic codes, inhibit the translation of messenger RNAs, interfere with the proofreading during translation, and thus inhibit the protein synthesis. The commonly used aminoglycosides include streptomycin, kanamycin, amikacin, isepamicin, and paromomycin. The mechanism of action of amikacin is to introduce the aminohydroxyl butyryl chain into the streptamine section in kanamycin structure, which has higher in vitro anti-TB activity than kanamycin. Isepamicin, a conjugate of gentamicin B and kanamycin A, is effective for amikacin-resistant MTB strains despite its lower antibacterial activity compared with amikacin (22). Paromomycin, obtained from the culture fluid of Streptomyces, is used mainly for treatment of multidrug-resistant TB (MDR-TB) (23). Capreomycin, a drug of cyclic peptides with similar antibacterial mechanism as aminoglycosides, is mainly used for the re-treatment and treatment of drug-resistant TB.
Oxazolidinones
The mechanism of action of linezolid, an oxazolidinone compound, is to inhibit bacterial protein synthesis. It exerts its antibacterial effect through combining with the 30S subunit on the interface near the bacterial 50S subunit and blocking the formation of the 70S initial complex. It is a new class of wholly synthetic antibacterial agents. Its target sites include 23SrRNA, ribosomal proteins L4 and L22, ERM-37 methyl transferase and whiB7 regulator protein, etc(24,25). Linezolid has good activity for MTB and mycobacterium avium without serious adverse reactions. After a series of structural modifications, synthetic PNU-100480 has obtained higher antibacterial activity than linezolid and no cross-resistance to first-line drugs, and therefore has became a promising drug for TB (26). Many countries are carrying out in-depth investigation into such antibacterial drugs. It has been found that novel oxazolidinone is substituted by compounds with stronger activities, such as PNU-172576, PNU-176665, pyrazinoindole, oxazinoindole or thioureas (27).
Macrolides
Macrolides include erythromycin, roxithromycin, azithromycin, and clarithromycin. Its antibacterial mechanism of action is to reversibly bind with the 50S subunit of the intracellular ribosome and thus interfere with the synthesis of bacterial protein. These drugs can not only inhibit the proliferation of bacteria but also affect the immunoregulation and inflammatory factors. Li et al. (28) measured the in vitro minimum inhibitory concentration (MIC) of six macrolides against 20 mycobacteria and concluded that macrolide antibiotics can be options for the clinical treatment of mycobacterial diseases. Their study laid a theoretical basis for the effective treatment of TB.
Nitroimidazoles
PA-824 and OPC-67683 are derived on the basis of CGI-17341. PA-824 is now under the phase II clinical trial with an MIC of 0.015-0.025 μg/mL, whereas OPC-67683 is under the phase II clinical trial, with an MIC of 0.006-0.024 mg/L against MTB (29,30). These drugs have a dual mechanism of action to inhibit the synthesis of both lipids and proteins on cell wall, which not only have antibacterial activity against compound bacteria of MTB, with strong bactericidal effect in the idiophase, non-idiophase, or persisting bacilli, but also have antibacterial activity against drug-sensitive and drug-resistant strains, without cross-resistance with conventional anti-TB drugs.
Inhibiting the metabolism of nucleic acid
Rifamycin derivatives
The main mechanism of action of rifampicin for MTB is as follows: it is bound firmly with the b subunit of bacterial DNA-dependent RNA polymerase to inhibit bacterial RNA synthesis and prevent the enzyme to be ligated to DNA, thereby blocking RNA transcription and terminating DNA and protein synthesis (31). In recent years, several rifamycin derivatives [rifapentine, rifalazil, rifabutin, and benzoxazine rifamycin-1648 (KRM-1648)] with anti-TB activity were obtained after the transformation of rifampicin aromatic proton C-3 and C-4, which can play a certain role in the treatment of drug-resistant PTB.
Quinolones
The quinolones can act on MTB DNA gyrase to prevent DNA replication and transcription and thus exert their antibacterial activities. They can be used to treat various drug-resistant TB. Gatifloxacin and moxifloxacin are superior to levofloxacin, ofloxacin and ciprofloxacin in terms of MIC (32). As the 4th generation fluoroquinolone, moxifloxacin has chemical structure remarkably different from other fluoroquinolones, in which the methoxyl group is introduced in its 8th carbon atom. Moxifloxacin can be metabolized via two pathways (sulphonating; combination with glucuronic acid) mainly into N-sulfates (M1) and acyl glucuronides (M2), rather than by the CYP450 pathway (33).
Phenazines
Phenazines include thioridazine, chlorpromazine, thioridazine, and clofazimine. As a phenothiazine analogue, thioridazine can play its role by elevating the lethality of MTB in non-killing macrophages and suppressing the effect of bacterial efflux pump (34). Chlorpromazine can preferably slow the growth of MTB in vitro and be effective for both drug-sensitive and resistant bacteria; thioridazine can improve the tolerance following clinical administration of TB, while its therapeutic effects on human TB remain to be further clarified. As a weak inhibitor of CYP3A4, clofazimine can not only inhibit MTB by suppressing transcription through its binding with mycobacterial DNA, but also defer rifampicin absorption and extend the duration of Cmax (peak plasma concentration), which is still to be further investigated clinically (35).
Pyrazinoic acids
Pyrazinamide can play its in vivo germicidal role after the pyrazinamidase is converted into an active form of pyrazine acid. It can discharge bacteria via the bacterial efflux system. Pyrazine acids can be protonated in the extracellular environment, and then re-enter MTB to release protons and cause fatal damage to the cell membrane (36). Another study has confirmed that pyrazine acids can bind the ribosomal protein S1 vital for MTB and block this protein-encoding MTB DNA, thereby preventing MTB to produce other proteins to sustain their survival. Thus, pyrazinamide can usually shorten the courses of treatment from 9-12 months to only several months (37).
Ethambutol
Ethambutol inhibits bacterial growth via interfering with RNA synthesis. Ethambutol can suppress arabinosyl transferase by inhibiting MTB Mt-EmbA and Mt-EmbB.
P-amino salicylic acids/p-aminosalicylic acid isoniazid
Similar to p-aminobenzoic acids, this class of drugs can destruct MTB folate metabolism through the competitive inhibition of MTB folate synthesis.
Diarylquinoline compounds
R207910 (TMC207) can inhibit bacterial ATP synthesis to deplete ATP and block the synthesis of ATP, and thus exert its bactericidal effect. This brand new anti-TB drug with this feature is now under the phase II clinical trial. Animal studies have shown that it has superior efficacy to the existing anti-TB drugs; it is more effective, faster, and less toxic (38). In mouse experiments, the combination of R207910+RFP+PZA or R207910+INH+PZA accelerates the bactericidal rate; after two months of treatment, MTB turned negative in all mice (39).
Interventional therapy
Interventional therapy is a new therapeutic approach evolving in recent 20 years, bringing new directions for the treatment of MDR-TB, endobronchial tuberculosis (EBTB), and massive hemoptysis caused PTB.
Bronchoscopy
Bronchoscopy has been widely applied in recent years. Literature has demonstrated the effectiveness of bronchoscopic interventional therapy. Touota et al. (30) infused anti-TB drugs into PTB cavity via bronchoscopy and added bronchodilators, protein dissolving agents, and dexamethasone, as appropriate, achieving good effectiveness in sputum negative conversion, foci absorption, and cavity closure. Ding et al. (40) retrospectively analyzed 149 patients who had undergone bronchoscopic balloon dilatation for tubercular bronchial stenosis and found that the airway diameter was increased to a varying extent immediately after balloon dilatation: airway diameter in the stenosis section increased from (2.7±1.4) to (6.8±2.0) mm after dilatation; it was still (6.4±1.7) and (6.3±2.3) mm, respectively, 3 months and 12 months after dilatation, with a success rate of 93.3% (139/149), a failure rate of 6.7% (10/149), and a serious complication rate of only 4.0% (6/149). Li et al. (41) found that, in patients with severe tubercular main bronchial stenosis, stent implementation was superior to bronchoscopic high-frequency electrocoagulation, balloon dilatation, cryotherapy, or other conventional interventional approaches; however, they also pointed out that follow-up should be enhanced as the re-stenosis rate was high, especially 6 months after stent placement.
Percutaneous needle lung biopsy
Tang et al. (42) have found that, after CT-guided percutaneous needle lung biopsy for the infusion of drugs among 66 patients with MDR cavitary PTB, the sputum negative conversion rate (70%), focus absorption rate (73.3%), and cavity closure rate (50%) were all significantly higher in the interventional therapy group than in the chemotherapy alone group. In addition, Zheng et al. (43) found that the application of central venous catheter in the interventional therapy for PTB treatment promoted the improvement of symptoms and ameliorated the patient's quality of life.
Bronchial artery embolization
Bronchial artery embolization is an effective approach for the treatment of massive hemoptysis. The Seldinger method was applied to perform femoral artery puncture, infusing the small gelatin sponge particles softened by immersion in the contrast agent into the bronchial arteries at the hemoptysis site to embolize the bronchial arteries for treatment of massive hemoptysis.
Pulmonary artery embolization
The incidence of pulmonary artery-derived hemoptysis is high in patients with chronic PTB with cavity or aspergilloma-dominant pulmonary infectious diseases. A hematoma forms after pulmonary artery damage, destruction of elastic fibers of the vessel wall and vascular re-rupture which generates a pulsatile hematoma with the hematoma that is organized to form the outer wall. The interior surface of the hematoma cavity is the intima extended from artery intimal cells. Thus, pulmonary artery pseudoaneurysm (PAPA) develops, with the indications of pulmonary embolization.
Surgical treatment
In early 1900s, various surgical approaches including collapse therapy, thoracoplasty, and pulmonary resection have been successively used in clinical practice, which greatly reduced the mortality caused by active TB infection.
Patients with TB are often complicated by lobar necrosis and multiple cavitary lesions with thick wall, making it difficult for immune factors, immunocytes, and drugs to infiltrate into the lesions to kill MTB. Surgical resection combined with chemotherapy can effectively remove the lesion and improve the MTB negative conversion rate and cure rate. Li et al. analyzed 217 cases of cavitary PTB after surgical treatment and found that their cure rate was 97.1% and sputum negative conversion rate, 93.6% (44). Nonetheless, the contraindications should be strictly evaluated to reduce the risk of surgery.
Immunotherapy
Immunotherapy-assisted drug therapy of TB can improve the cure rate of MDR-TB, shorten the duration of treatment, and ameliorate patients’ immunity. Its indications include the initial treatment or retreatment of TB complicated by immune dysfunction, severe PTB, drug-resistant or multidrug-resistant TB, non-reactive TB, or TB with concurrent immune dysfunction. Its targets of action involve the following mechanisms: motivation of the anti-TB antibodies; usage of MTB antigen to improve the protective function of TH1 cells or increase TH1 cytokines; interference with the inflammatory response; targeted immunosuppressive pathways; and targeted cell activation/proliferation pathways. Currently, many biological products with immunomodulatory functions have been applied in the immunotherapy of TB infection.
Cytokines
As ancillary drugs, IL-2, IFN-γ, and IL-7 can increase the cure rate of MDR-TB, shorten the course of treatment, ameliorate patients’ immunity, and increase the body’s clearance rate of MTB (45). Dawson R et al. have shown that aerosol inhalation of IFN-γ or other ancillary drugs can not only reduce symptoms such as night sweating or fever but also increase the clearance of MTB (46). IFN-γ reduces the production of IL-17, which can stimulate the aggregation and activation of neutrophils. Nandi B argued that the increase in the number of neutrophils can result in immune non-responsiveness in Th1 cells (IFN-γ-deficient reaction). These data suggest that IFN-γ can play multiple roles in the treatment of TB (47). Many other approaches have also been used to inhibit excessive inflammation. For instance, intravenous immunoglobulin (IVIG) is used as an immunotherapy for TB, which might be related to the variable region (V) of the Fab fragment and the Fc fragment, whereas high-concentration exogenous immunoglobulin may compete for endogenous antibodies in circulation (48-50).
Therapeutic vaccines
Bacillus Calmette-Guérin (BCG) immunotherapy can enhance the Th1 immune pathways and, particularly, the cytotoxic immune pathways. It can also increase the efficacy of clinical treatment of TB and its bacteriological cure rate, and meanwhile reduce the 5-year recurrence rate and the incidence of MDR-TB. Special attention should be given to the choice of immunotherapy timing, as when used alone or in a premature way may aggravate the pathologic injury (51,52). Other mmunotherapies for mycobacteria include Mycobacterium smegmatis bacterins and Mycobacterium phlei preparations (tradename UTILIN’S) can all achieve satisfactory results in enhancing Th1-type immunity and improving the clinical outcomes of TB. Treating using inactivated vaccine or Mycobacterium extract vaccine such as Mycobacterium vaccae vaccine and BCG polysaccharide and nucleic acid injections can promote the proliferation of monocyte-macrophage system, enhance the phagocytosis and digestion of macrophage, improve the ability of macrophages to produce NO or H2O2, significantly increase T lymphocyte and natural killer cell function in vivo, activate a variety of lymphokines released by T cells, and elevate the levels of IL-2 and IL-2 receptor expression and IFN-γ induction; when used in combination with chemotherapy, the therapy can result in weight gain among patients with TB, speed up sputum conversion, focus absorption, and cavity narrowing or closure, shorten short-course chemotherapy, and improve the efficacy of the combined chemotherapy. In terms of gene vaccine, many MTB DNA vaccines such as hsp65, hsp70, Ag85A, Ag85B and MPT64 DNA vaccines, have been found to be effective when used as auxiliary treatment. DNA-hsp65 achieves its immune therapeutic effects by inhibiting Th2 cytokines and regulating the expression of inflammatory cytokines including IFN-γ, IL-17, lymphotoxin-α, TNF-α, IL-6, TGF-beta, iNOS and Foxp3 (53-56). The expression of Ag85B-ESAT-6 fusion protein in Mycobacterium smegmatis (M.s) can significantly improve the immunogenicity of M.s and is capable of stimulating the body to produce the immune response that is conducive to the anti-Mtb infections in mice. Thus, it is endowed with certain research prospects as a novel candidate vaccine for TB (57). Fragmental MTB vaccine (RUTI) is now in the phase of clinical trials; compared with chemotherapy alone, it can shorten the duration of chemotherapy.
Traditional Chinese Medicine (TCM) therapies
Many recent studies have shown that TCM therapy can be bacteriostatic and bactericidal, reduce viral resistance, and improve patients’ immunity.
Single traditional Chinese medicine
Studies have shown that garlicin can not only inhibit MTB protein synthesis but also inhibit bacterial rotamase, thus preventing DNA replication and degradation and ultimately resulting in MTB death (58). Pulsatilla chinensis can inhibit MTB in vitro while enhancing immune function. It has also been found that Pulsatilla chinensis can suppress the hepatotoxicity of rifampicin and isoniazid and thus plays a protective role for the liver. Combined use of Pulsatilla chinensis, rifampin, and isoniazid can ensure their original efficacies and meanwhile eliminate their side effects (59). Some authors have confirmed through in vitro bacteriostatic experiments that berberine can inhibit MTB in vitro, and the different activities of berberine are closely correlated with its concentrations. Cordyceps sinensis contains a variety of vitamins and trace elements, as well as 19 kinds of amino acids including asparaginic acid and adenosine, which are helpful to enhance the activities of lymphocytes and DNA repair. When used in combination with chemotherapeutic drugs, it can increase the patients’ tolerance to chemotherapeutic drugs as well as the immunity, relieve their symptoms including night sweating and fatigue, inhibit the toxic side effects of chemotherapeutic drugs, and, in particular, prevent liver damage. Thus, it can be an ideal adjunctive drug for the treatment of TB (60). Sophora flavescens can separate the monomeric alkaloid matrine, which can effectively improve the body’s immune system, resist inflammation, viruses, and tumors, and protect the liver (61).
Compound preparations
It has been proved that Feitai Capsule, Shenling Baizhu Powder, Compound Astragalus Capsule, anti-phthisis capsule and other Chinese patent drugs used in conjunction with chemotherapeutic drugs can promote the sputum negative conversion rate, cavity closure rate, and lesion absorption rate; meanwhile, they can also alleviate the toxic effects of anti-TB drugs, rapidly improve TB symptoms, and thereby increase the efficacy (62-65).
Today, an increasing number of therapies have been available for the treatment of TB, although anti-TB drugs remain the fundamental approaches. In fact, the rational use of anti-TB drugs plays an important role in improving the efficacy of TB and reducing side effects. Due to the prevalence of MDR-TB, it is of extreme urgency to find more efficient antimicrobial agents or (and) bactericidal agents. Meanwhile, further investigation on the rational combination of different therapeutic strategies is still warranted.
Acknowledgements
We appreciate Prof. Xiao Heping for his instructions and guidance.
Disclosure: The authors declare no conflict of interest.
References
- 1.Wang J, Zhang HB, Zhou JP, et al. Advances in new anti-tuberculosis targets and related drugs. Zhong Guo Yao Ke Da Xue Xue Bao 2012;43:1-8 [Google Scholar]
- 2.Gupta R, Lavollay M, Mainardi JL, et al. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 2010;16:466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Z, Barletta RG. Roles of Mycobacterium smegmatis D-alanine: D-alanine ligase and D-alanine racemase in the mechanisms of action of and resistance to the peptidoglycan inhibitor D-cycloserine. Antimicrob Agents Chemother 2003;47:283-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu H, Xin Y, Dong X, et al. An rmlA gene encoding d-glucose-1-phosphate thymidylyltransferase is essential for mycobacterial growth. FEMS Microbiol Lett 2007;275:237-43 [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Pan F, McNeil M.Formation of dTDP-rhamnose is essential for growth of mycobacteria. J Bacteriol 2002;184:3392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin AG, Goude R, Shi L, et al. EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology 2008;154:240-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Scherman MS, D’Haeze W, et al. Identification and active expression of the Mycobacterium tuberculosis gene encoding 5-phospho-{alpha}-d-ribose-1-diphosphate: decaprenyl-phosphate 5-phosphoribosyltransferase, the first enzyme committed to decaprenylphosphoryl-d-arabinose synthesis. J Biol Chem 2005;280:24539-43 [DOI] [PubMed] [Google Scholar]
- 8.Alderwick LJ, Molle V, Kremer L, et al. Molecular structure of EmbR, a response element of Ser/Thr kinase signaling in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2006;103:2558-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu H, Xin Y, Dong X, et al. An rmlA gene encoding d-glucose-1-phosphate thymidylyltransferase is essential for mycobacterial growth. FEMS Microbiol Lett 2007;275:237-43 [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Pan F, McNeil M.Formation of dTDP-rhamnose is essential for growth of mycobacteria. J Bacteriol 2002;184:3392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderwick LJ, Seidel M, Sahm H, et al. Identification of a novel arabinofuranosyltransferase(AftA) involved in cell wall arabinan biosynthesis in Mycobacteriumtuberculosis. J Biol Chem 2006;281:15653-61 [DOI] [PubMed] [Google Scholar]
- 12.Sidel M, Alderwick KJ, Birch HJ. Identification of a novel arabinofuranosyltranferase AItB involved in a terminal step of cell wall arabinan biosynthesis in corymebacterianeae, such as corynebacterium glutamicum and Mycubacterium tuberculos. Bio Chem 2007;282:14729-40 [DOI] [PubMed] [Google Scholar]
- 13.Seidel M, Alderwick LJ, Birch HL, et al. Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J Biol Chem 2007;282:14729-40 [DOI] [PubMed] [Google Scholar]
- 14.Birch HL, Alderwick LJ, Bhatt A, et al. Biosynthesis of mycobacterial arabinogalactan: identification of a novel alpha(1-->3) arabinofuranosyltransferase. Mol Microbiol 2008;69:1191-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean KJ, Marshall KR, Richmond A, et al. Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology 2002;148:2937-49 [DOI] [PubMed] [Google Scholar]
- 16.Jackson CJ, Lamb DC, Kelly DE, et al. Bactericidal and inhibitory effects of azole antifungal compounds on Mycobacterium smegmatis. FEMS Microbiol Lett 2000;192:159-62 [DOI] [PubMed] [Google Scholar]
- 17.Ahmad Z, Sharma S, Khuller GK. Azole antifungals as novel chemotherapeutic agents against murine tuberculosis. FEMS Microbiol Lett 2006;261:181-6 [DOI] [PubMed] [Google Scholar]
- 18.Ahmad Z, Sharma S, Khuller GK, et al. Antimycobacterial activity of econazole against multidrug-resistant strains of Mycobacterium tuberculosis. Int J Antimicrob Agents 2006;28:543-4 [DOI] [PubMed] [Google Scholar]
- 19.Ahmad Z, Sharma S, Khuller GK. Chemotherapeutic evaluation of alginate nanoparticle-encapsulated azole antifungal and antitubercular drugs against murine tuberculosis. Nanomedicine 2007;3:239-43 [DOI] [PubMed] [Google Scholar]
- 20.Ahmad Z, Pandey R, Sharma S, et al. Novel chemotherapy for tuberculosis: chemotherapeutic potential of econazole- and moxifloxacin-loaded PLG nanoparticles. Int J Antimicrob Agents 2008;31:142-6 [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Kim S, Yun MH, et al. Synthesis and antitubercular activity of monocyclic nitroimidazoles: insights from econazole. Bioorg Med Chem Lett 2011;21:1515-8 [DOI] [PubMed] [Google Scholar]
- 22.Lounis N, Ji B, Truffot-Pernot C, et al. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis in mice? Antimicrob Agents Chemother 1997;41:607-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanyok TP, Reddy MV, Chinnaswamy J, et al. In vivo activity of paromomycin against susceptible and multidrug-resistant Mycobacterium tuberculosis and M. avium complex strains. Antimicrob Agents Chemother 1994;38:170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang SJ, Xiao HP, Zhang Q. Research development on chemotherapy of multidrug-resistant tuberculosis. Zhong Hua Jie He He Hu Xi Za Zhi 2009,32:617-620 [PubMed] [Google Scholar]
- 25.Cremades R, Rodríguez JC, Garcia-Pachón E, et al. Interaction between linezolid and Mycobacterium tuberculosis in an experimental in vitro model. APMIS 2011;119:304-8 [DOI] [PubMed] [Google Scholar]
- 26.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin,ofloxacin,sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 2007;51:576-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007;45:1001-7 [DOI] [PubMed] [Google Scholar]
- 28.Coyne KM, Pozniak AL, Lamorde M, et al. Pharmacology of second-line antituberculosis drugs and potential for interactions with antiretroviral agents. AIDS 2009;23:437-46 [DOI] [PubMed] [Google Scholar]
- 29.de Jonge MR, Koymans LH, Guillemont JE, et al. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins 2007;67:971-80 [DOI] [PubMed] [Google Scholar]
- 30.Toyota E, Kobayashi N, Takahara M, et al. Clinical investigation on endobronchial tuberculosis. Kekkaku. 1999;74:347-51 [PubMed] [Google Scholar]
- 31.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001;40:327-41 [DOI] [PubMed] [Google Scholar]
- 32.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin,ofloxacin,sparfloxacin,and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 2007;51:576-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007;45:1001-7 [DOI] [PubMed] [Google Scholar]
- 34.Amaral L, Viveiros M.Why thioridazine in combination with antibiotics cures extensively drug-resistant Mycobacterium tuberculosis infections. Int J Antimicrob Agents 2012;39:376-80 [DOI] [PubMed] [Google Scholar]
- 35.Mehta J, Gandhi IS, Sane SB, et al. Effect of clofazimine and dapsone on rifampicin (Lositril) pharmacokinetics in multibacillary and paucibacillary leprosy cases. Lepr Rev 1986;57:67-76 [PubMed] [Google Scholar]
- 36.Zimic M, Fuentes P, Gilman RH, et al. Pyrazinoic acid efflux rate in Mycobacterium tuberculosis is a better proxy of pyrazinamide resistance. Tuberculosis (Edinburgh, Scotland) 2012;92:84-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 2011;333:1630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009;360:2397-405 [DOI] [PubMed] [Google Scholar]
- 39.de Jonge MR, Koymans LH, Guillemont JE, et al. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins 2007;67:971-80 [DOI] [PubMed] [Google Scholar]
- 40.Ding WM, Wang JP, Fu Y, et al. The clinical value of balloon dilatation through flexible bronchoscope in the management of tracheobronchial stenosis in 149 cases of endobronchial tuberculosis. Jie He Bing Yu Xiong Bu Zhong Liu 2010;39:250-5 [PubMed] [Google Scholar]
- 41.Li Y, Yao XP, Li H, et al. Therapeutic efficacy analysis of bronchoscopic interventional therapy on severe tuberculous main bronchial stenosis complicated with unilateral atelectasis. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:454-8 [PubMed] [Google Scholar]
- 42.Tang SJ, Xiao HP, Li Hong, et al. The short-term and long-term clinical treatment outcome of CT-guided percutaneous lung centesis administration in treatment of cavernous multi-drug resistant pulmonary tuberculosis. Zhong Guo Fang Lao Za Zhi 2009;31:40-5 [Google Scholar]
- 43.Zheng Z, Yang KY, Wang YL, et al. Clinical study of interventional treatment under CT-guided percutaneous lung centesis indwelling central venous catheter administration in treatment of cavitary multi-drug resistant pulmonary tuberculosis. Zhong Guo Dang Dai Yi Yao 2010;21:15-7 [Google Scholar]
- 44.Li XP, Wu JF, Bai Y, et al. Surgical operation in the treatment of 217 cases of pulmonary tuberculosis with cavitation. Lin Chuang Fei Ke Za Zhi 2011;16:84-5 [Google Scholar]
- 45.Uhlin M, Andersson J, Zumla A, et al. Adjunct immunotherapies for tuberculosis. J Infect Dis 2012;205:S325-34 [DOI] [PubMed] [Google Scholar]
- 46.Dawson R, Condos R, Tse D, et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PloS One 2009;4:e6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med 2011;208:2251-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shehata N, Palda VA, Meyer RM, et al. The use of immunoglobulin therapy for patients undergoing solid organ transplantation: an evidence-based practice guideline. Transfus Med Rev 2010;24:S7-27 [DOI] [PubMed] [Google Scholar]
- 49.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008;26:513-33 [DOI] [PubMed] [Google Scholar]
- 50.Ballow M.The IgG molecule as a biological immune response modifier:mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol 2011;127:315-23;quiz 24-25 [DOI] [PubMed] [Google Scholar]
- 51.Lei JP, Xiong GL, Hu QF, et al. Immunotherapeutic efficacy of BCG vaccine in pulmonary tuberculosis and its preventive effect on multidrug-resistant tuberculosis. Zhonghua Yu Fang Yi Xue Za Zhi 2008;42:86-9 [PubMed] [Google Scholar]
- 52.Lei JP, Peng YX, Guo L, et al. The different influence of BCG vaccination to the treatment effect of rats with tuberculosis and to the peripheral blood T lymphocyte subset at different period. Zhong Guo Fang Lao Za Zhi 2006;28:404-407 [Google Scholar]
- 53.Hu JG, Hu XW. The effect of anti-tuberculosis drug combined with utilin’s on pulmonary tuberculosis. Ji Bing Kong Zhi Za Zhi 2003;7:37-9 [Google Scholar]
- 54.Xu M, Chen BW, Shen XB, et al. The immune modulatory function of M.smegmatis preparation in animals with different immune status. Zhong Hua Wei Sheng Wu He Mian Yi Xue Za Zhi 2005;25:752-5 [Google Scholar]
- 55.Wang Q, Zhang J, Huang SP, et al. Immunogenicity of the recombinant M.smegmatis expressing Ag85B-ESAT-6 fusion protein of M.tuberculosis in mice. Zhong Guo Yao Fang 2008;19:838-841 [Google Scholar]
- 56.Song MJ, Wang LM, Zhang XL, et al. Immunotherapeutic effect of polysaccharide nuleic acid fraction of BCG on retreated pulmonary tuberculosis. Lin Chuang Fei Ke Za Zhi 2008;13:442-3 [Google Scholar]
- 57.Wang P, Wang LM, Zhang W, et al. Immunogenicity of the recombinant M.smegmatis expressing Ag85B-ESAT-6 fusion protein of M.tuberculosis in mice. Zhong Guo Fang Lao Za Zhi 2012;3:19-23 [Google Scholar]
- 58.Liu YL, Liu ZP. Observing the Effect of Alltride Enteric-Coated Soft Capsules Combined With Anti-tuberculosis Medicine on Pulmonary Tuberculosis. Zhong Guo Yi Liao Qian Yan 2008,;3:23-5 [Google Scholar]
- 59.Wang SY, Wang XL, Liu MM. Experimental study of effects of Chinese bulbul extracts againsting Mycobacterium Tuberculosis in vitro. Shi Zhen Guo Yi Guo Yao 2011;22:2965-6 [Google Scholar]
- 60.Kuang TJ, Dong M, Song P, et al. In vitro antibacterial effects of berberine on mycobacterium tuberculosis. Zhong Guo Zhong Yao Za Zhi 2001;26:867-8 [Google Scholar]
- 61.Chen CS, Liang Y. Analysis of the potency of matrine in the inhibition of mycobacteritum tuberculosis. Shou Du Yi Yao 2006;13:44 [Google Scholar]
- 62.Gao MQ, Zhu LZ, Yuan SL. The observation of short-term efficacy and safety of Chinese herbal medicine Feitai Capsule adjunctive therapy for retreatment of pulmonary tuberculosis. Zhong Hua Jie He He Hu Xi Za Zhi 2006;29:134-6 [Google Scholar]
- 63.Hao XP, Yang QF. Observation of clinical effect of Shenling Baizhu Powder on treatment of gastrointestinal side effect of antituberculosis drugs. Xin Zhong Yi 2003;35:34-5 [Google Scholar]
- 64.Zhang LH, Ma XQ. Clinical Study of drug-resistant pulmonary tuberculosis treated by combination of anti-tuberculosis chemicals and Compound Astragalus Capsule. Lin Chuang Fei Ke Za Zhi 2005;10: 665-6 [Google Scholar]
- 65.Li G.Research of effects on anti-pulmonary tuberculosis of anti tuberculosis capsule in vitro and in vivo. Zhong Guo Shi Yong Yi Yao, 2010;5:5-7 [Google Scholar]


