Abstract
Purpose
To compare the effect of using fibrin glue or 10-0 nylon sutures on the clinical outcome of patients undergoing pterygium excision and conjunctival autografting.
Methods
We retrospectively reviewed the medical records of 52 eyes from 46 patients who underwent pterygium excision and conjunctival autografting and were followed up for more than 3 months. The operation duration, postoperative inflammation, complications, and recurrence rates were compared between groups of 20 patients (22 eyes) for whom fibrin glue was used (fibrin glue group) and 26 patients (30 eyes) for whom suturing was performed with 10-0 nylon (suture group) in pterygium excision and conjunctival autografting.
Results
The operation duration was 27.71 (5.22) minutes in the fibrin glue group and 43.30 (8.18) minutes in the suture group (p = 0.000). Seven days after the operation, the fibrin glue group showed milder conjunctival inflammation than the suture group (p = 0.000). Postoperative complications and corneal recurrence rates were not statistically different between the two groups.
Conclusions
The use of fibrin glue in pterygium excision with conjunctival autografting is likely to be a more effective, safer procedure than suturing.
Keywords: Conjunctival autograft, Fibrin glue, Pterygium excision
Pterygium is a wing-shaped fibrovascular tissue that has proliferated onto the cornea. Ultraviolet radiation and hot, dusty, windy, dry, smoky environments are regarded as risk factors for pterygium [1-3]. Surgical removal is the treatment of choice, and pterygium removal with conjunctival autografting has been considered the best and most suitable approach that can lower the recurrence rate of pterygium [4].
Conjunctival autografting using fibrin glue is an emerging theme in pterygium surgery in recent years. Many reports, dating back to the early 2000s, have stated that operation duration and postoperative symptoms such as pain, photophobia, and foreign body sensation decreased when fibrin glue was used as a substitute for suturing in pterygium surgery [5-9].
In this study, we retrospectively compared the efficacy, postoperative inflammation, complications, and recurrence rates between a group of Asian patients for whom fibrin glue was used (fibrin glue group) and that for whom 10-0 nylon was used (suture group) in pterygium excision and conjunctival autografting.
Materials and Methods
We retrospectively reviewed the medical records of patients who underwent pterygium excision and conjunctival autografting between January 2008 and May 2010. The inclusion criteria were as follows: 1) a follow-up period of more than 3 months after surgery and 2) no immune-related disease, ocular surface disease, or eyelid disease. Patients with recurrent pterygium, which is accompanied by symblepharon or limitation of duction, were excluded from the study. Informed consent was obtained from all patients. The study protocol was approved by the institutional review board at Seoul National University Hospital and was in accordance with the Helsinki Declaration. The study sample finally consisted of 46 patients (52 eyes): 20 patients (22 eyes) in the fibrin glue group and 26 patients (30 eyes) in the suture group. Two records were excluded because the patients were lost to follow-up.
Best-corrected visual acuity (BCVA), slit-lamp examination, and determination of intraocular pressure were performed after the operation. The degree of growth of the pterygium was evaluated in terms of the following 2 parameters: the length of the invading head from the limbus and density of the pterygium alone. The length of the invading head was calculated using a slit lamp, and the density of the pterygium was graded according to the system used by Tan et al. [10] (type 1: atrophic, type 2: mild inflammation, and type 3: moderate/severe inflammation or showing active growth). The conjunctival autograft was sutured with 10-0 nylon for patients who underwent surgery between January 2008 and October 2009, and was attached using fibrin glue (Greenplast; Greencross Corp., Yongin, Korea) for those who underwent surgery between November 2009 and May 2010.
Surgical techniques
A single surgeon (KMK) performed all the surgeries. Topical anesthesia using 0.5% proparacaine HCl (Alcaine; Alcon Laboratories, Fort Worth, TX, USA) was applied and 2% lidocaine was injected into the pterygium body. The pterygium was dissected from the apex using a straight beaver blade. The pterygium body and underlying fibrovascular tissues were delineated from the conjunctiva and removed. A 1-mm sponge soaked with 0.02% mitomycin C solution was placed onto the deep fibrovascular bundle in the medial canthus for 2 minutes, and the applied area was irrigated with 40 mL of balanced salt solution. The upper conjunctiva was harvested and placed into the lesion. The grafted tissue was continuously sutured with 10-0 nylon, including 4 interrupted sutures, or it was sealed with fibrin glue. The sutures with 10-0 nylon were buried. In the fibrin glue group, each syringe of fibrin glue, which is filled with clottable plasma protein or thrombin solution respectively, was prepared with a 23-gauge needle. These components were applied simultaneously to the exposed sclera, using as little as possible, by pushing the double syringe device at the same time. Both wound margins were held together with tooth forceps to ensure adhesion. Triamcinolone was injected into the inferior subconjunctival space for a total of 0.3 mL after the operation to reduce postoperative inflammation and prevent pterygium recurrence [11]. The operation duration was considered as the time from when the lid retractor was placed until its removal at the end of surgery. After surgery, all patients were prescribed topical prednisolone (Pred Forte; Allergan Corp., Irvine, CA, USA) and topical levofloxacin (Cravit; Santen, Osaka, Japan) 4 times daily for 2 weeks.
Postoperative follow-up
BCVA, slit-lamp examination, and determination of intraocular pressure were performed at 1, 7, 14, and 28 days and at 2 to 3 months after the operation. In the suture group, 10-0 nylon was stitched at the edges at 14 days after the operation, and the anchoring suture along with the limbus, which was supposed to reduce recurrence, was removed at 28 days after the operation. The anterior segment was photographed at 7 and 28 days after the operation, and corneal topography was examined at 28 days after the operation.
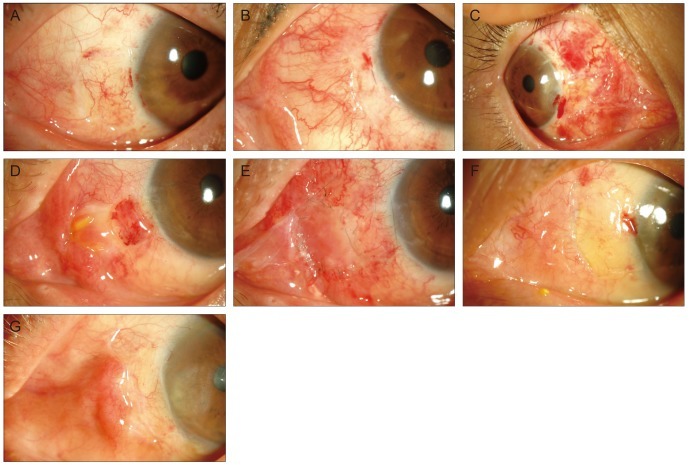
Two ophthalmologists, who were blinded to which group the anterior segment photographs were from, evaluated the postoperative inflammation of photographs. The degree of postoperative inflammation was classified into 3 grades: mild, moderate, and severe inflammation (Fig. 1A-1C). Postoperative complications were examined, including dehiscence, dislodge, ridge, sub-graft hemorrhage, chemosis, necrosis, contracture, and granuloma. An additional stitch was taken in the case of a dislodged graft, and severe granulomas were surgically removed when medication could not reduce the granuloma. Recurrence of pterygium was defined as newly developed fibrovascular tissue invading the cornea to cross the limbus.
Fig. 1.
(A-C) The standard anterior segment photographs showing inflammation at 1 week after the operation (A, mild inflammation; B, moderate inflammation; C, severe inflammation). (D) Photograph of pyogenic granuloma observed in a 53-year-old woman at 1 month after she underwent pterygium excision and conjunctival autografting. (E) Photograph at 3 days after the operation when the pyogenic granuloma was removed and amniotic membrane was transplanted. (F) Photograph of dehiscence in a 57-year-old woman at 7 days after she underwent pterygium excision and conjunctival autografting. (G) Photograph of a ridge in a 71-year-old man at 28 days after he underwent pterygium excision and conjunctival autografting in his left eyes.
Statistical analyses
Data were expressed as the mean (standard deviation). Chi-square test, independent t-test, paired t-test, Mann-Whitney U-test, and Wilcoxon signed rank test were used to analyze the data. BCVA (decimal visual acuity) was transferred to the logarithm of the minimum angle of resolution (logMAR) to be analyzed. A p-value of less than 0.05 was considered statistically significant.
Results
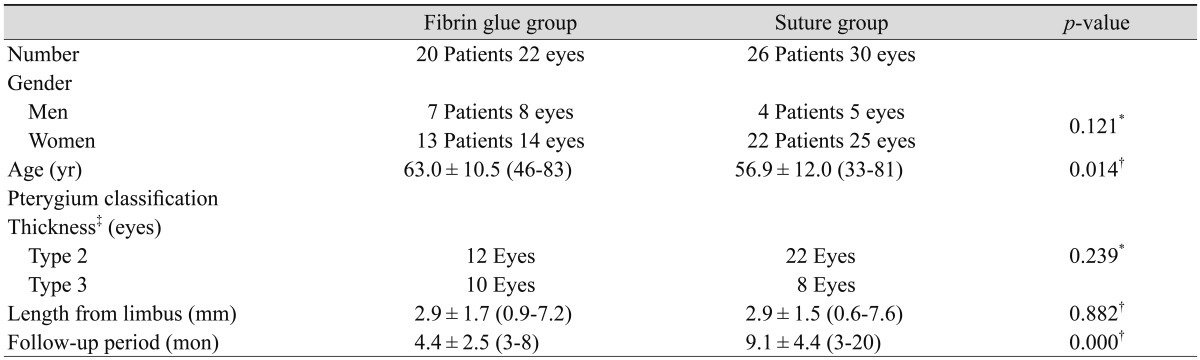
There were 7 men and 13 women in the fibrin glue group and 4 men and 22 women in the suture group. The mean age (SD) was 63.0 (10.5) years (range, 46 to 83 years) in the former and 56.9 (12.0) years (range, 33 to 81 years) in the latter. The mean head length of the pterygium from the limbus was 2.9 (1.7) and 2.9 (1.5) mm in the fibrin glue group and suture group, respectively (p = 0.882). In both groups, all the pterygiums corresponded to type 2 or type 3 (p = 0.239). The mean follow-up period was 4.4 (2.5) months (3 to 8 months) and 9.1 (4.4) months (3 to 20 months) in the fibrin glue group and suture group, respectively (p = 0.000) (Table 1).
Table 1.
Demographic data of patients in the fibrin glue and suture group
Values are presented as mean ± standard deviation.
*Tested using the chi-square test; †Tested using the Mann-Whitney U-test; ‡Grading of pterygium thickness by Tan et al. [10] (type 2: mild inflammation, type 3: moderate/severe inflammation or showing active growth).
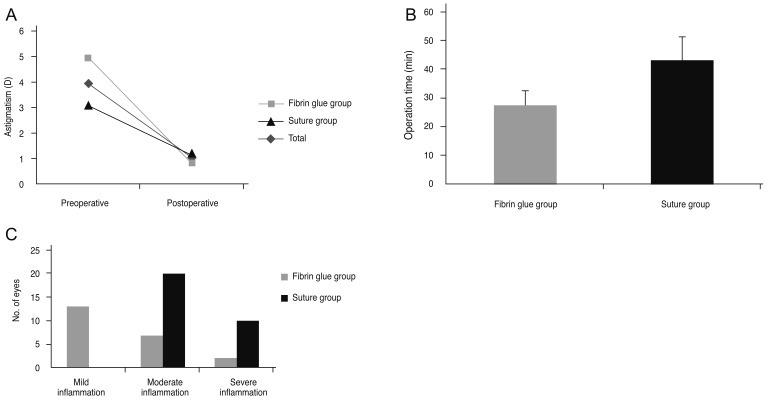
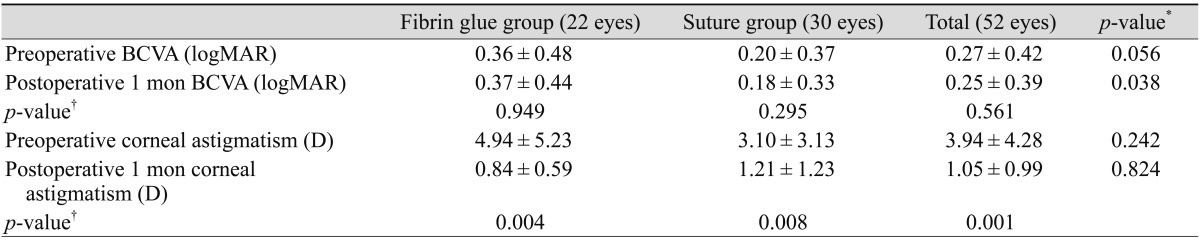
Preoperative BCVA (logMAR) was 0.27 (0.42), and BCVA at 1 month after the operation was 0.25 (0.39) in the entire group; the difference between these two parameters was insignificant (p = 0.561). However, corneal astigmatism was significantly reduced in the total group from 3.94 (4.28) diopter to 1.05 (0.99) diopter (p = 0.000) (Fig. 2A). The reduction in astigmatism was not significantly different between the two groups (Table 2). The operation duration was 27.71 (5.22) minutes in the fibrin glue group, which was remarkably shorter than that in the suture group (43.30 [8.18] minutes, p = 0.000) (Fig. 2B).
Fig. 2.
(A) Corneal astigmatism was reduced from 4.94 (5.23) diopter (D), 3.10 (3.13) D, and 3.94 (4.28) D to 0.84 (0.59) D, 1.21 (1.23) D, and 1.05 (0.99) D in the fibrin glue, suture, and total group, respectively, 1 month after pterygium surgery (p = 0.004, 0.008, and 0.000, Wilcoxon signed rank test). (B) Operation time was 27.71 (5.22) minutes in the fibrin glue group and 43.30 (8.18) minutes in the suture group, and the difference between these 2 values was statistically significant (p = 0.000, Mann-Whitney U-test). (C) In the fibrin glue group, 9 out of 22 eyes showed moderate to severe conjunctival inflammation, whereas in the suture group, all 30 eyes showed moderate to severe inflammation at 1 week after pterygium surgery (p = 0.000, chi-square test).
Table 2.
Ocular manifestations of patients in the fibrin glue and suture groups
Values are presented as mean ± standard deviation.
BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; D = diopter.
*Tested using the Mann-Whitney U-test; †Tested using the Wilcoxon signed rank test (individual groups) or paired t-test (total group).
At 7 days after the operation, 9 out of 22 eyes (40.9%) showed moderate to severe conjunctival inflammation in the fibrin glue group, whereas all 30 eyes (100%) in the suture group showed moderate to severe inflammation (p = 0.000) (Fig. 2C). After 21 days, conjunctival inflammation almost subsided in both groups, and the degree of conjunctival inflammation was not significantly different between the two groups.
The rate of complication, including dehiscence, ridge, proliferative granuloma, sub-graft hemorrhage, chemosis, dislodgement, and contracture, did not significantly differ between the two groups (Table 3). However, granulomas tended to occur more often in the fibrin glue group. In fact, one granuloma was so proliferative that it required intervention (Fig. 1D). Surgical removal of the granuloma and subsequent amniotic membrane transplantation made the irregular surface flat and did not show recurrence during the four months' follow up (Fig. 1E). In two other cases, the granulomas regressed with topical and systemic steroid treatment. Dehiscences were mainly observed between the nasal margin of the graft and the adjacent conjunctiva, but the gap was very narrow and linear, and all the surfaces were fully epithelized within one week and did not require intervention (Fig. 1F).
Table 3.
Complications associated with pterygium excision in the fibrin glue and suture groups
Values are presented as number (%).
*Tested using the chi-square test.
The recurrence rate of corneal pterygium within three months of the surgery was 4.55% (1 of 22 eyes) in the fibrin glue group and 20.0% (6 of 30 eyes) in the suture group; however, the difference in the recurrence rate was not statistically significant (p = 0.067).
Discussion
There are several kinds of surgeries that excise the pterygium and repair the conjunctiva. Among these, the excision of the pterygium with conjunctival autografting is considered to be the procedure of choice in terms of efficacy and long-term stability [4,12]. It was reported that the recurrence rate in the case of conjunctival autografting was much lower than that in the case of primary closure or amniotic membrane grafting [4]. Adjunctive therapies such as mitomycin C, β-irradiation, and excimer laser have also been used to decrease the recurrence rate of pterygium in spite of potentially sight-threatening side effects [13-15]. This is the basis for selecting conjunctival autografting with the use of mitomycin C as our surgical protocol.
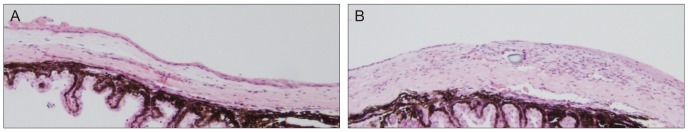
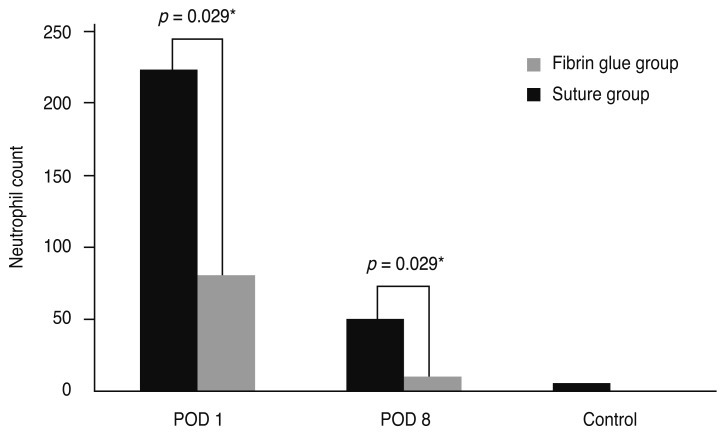
Fibrin glue is a blood-derived product which consists of a fibrinogen component and a thrombin component. It imitates the final stages of the coagulation cascade when two components are mixed. Recently, its spectrum of use in medicine is broad, including neurosurgery, plastic surgery, surgery in otolaryngology, and eye surgery [16-18]. In ophthalmology, fibrin glue is being used in strabismus surgery, corneal surgery, amniotic membrane transplantation, and conjunctival closure following pterygium [19-21] . In the early 2000s, fibrin glue was introduced to pterygium excision and conjunctival autografting as a substitute for suture. Using a questionnaire, Uy et al. [6] found that fibrin glue, when used for attaching conjunctival autografts, caused less postoperative discomfort than 10-0 nylon. Several other studies also reported similar results, but most of them used questionnaires to evaluate the degree of postoperative symptoms such as pain, photophobia, foreign body sensation, irritation, epiphora, and itching [5,7,8,22-24]. We supported these subjective findings of previous reports by evaluating an anterior segment photograph using an objective grading system. Srinivasan et al. [9] also used standardized slit lamp photographs and demonstrated that fibrin glue produced less postoperative inflammation than suture. Our data showed some discrepancy with that reported by Srinivasan et al. [9]; they reported no difference in inflammation between the fibrin glue or suture group at 1 week, but inflammation was reduced significantly in the fibrin glue group after 1 to 3 months. On the contrary, we reported significantly less inflammation in the fibrin glue group than suture group at 1 week, and no difference in inflammation between these groups at 1 month. This disparity may be explained by the use of different suture material in each study. Srinivasan et al. [9] used multiple interrupted 10-0 polyglactin sutures that were slowly resolved over time. This could be a potential source of chronic inflammation. On the other hand, we used non-absorbable 10-0 nylon, which can trigger inflammation in the earlier period before removal of the stitches. After the stitches were removed at 10 to 14 days after the operation, there was no suture-related side effect on inflammation, which explains the absence of clinical inflammation at 1 month in our study. Nevertheless, our study corresponded well with Srinivasan et al. [9] who stated that fibrin glue was less likely to induce inflammation than suture. We also found that our preliminary animal study using a mouse model showed significantly less inflammation in glued conjunctiva than in 10-0 nylon sutured conjunctiva (data are shown in the supplementary section, Fig. 1S and 2S), supporting our clinical outcome.
Fig. 1S.
Significantly less infiltration of inflammatory cells in the mouse (A) which underwent surgery using fibrin glue, compared to the mouse using the conventional suture method (B) (H&E, ×100).
Fig. 2S.
Postoperative inflammation. Neutrophil count infiltrating the wound was significantly lower in the fibrin glue group than the suture group at postoperative day 1 and 8, and two groups showed a similar degree of cellular infiltration after suture material was removed in the suture group. POD = postoperative day. *Mann-Whitney U-test.
The main constituent of fibrin glue is the same as that found in human beings, and hence it is less immunogenic. However, various pathogens such as parvovirus B19, human immunodeficiency virus, and Creutzfeldt-Jakob agent may be theoretically transmitted to human beings during surgery [25,26]. In thoracic surgery, there is a report stating that parvovirus B19 was clinically expressed after using fibrin glue [27]. However, there have been no reports describing postoperative infection after the use of fibrin glue in pterygium surgery.
Regarding other complications in glue-assisted pterygium surgeries, previous reports did not present much of a difference between glue-assisted and conventional pterygium surgeries [7,28]. However, we found that dehiscence was greater in glue-assisted pterygium surgery, albeit not significantly greater. Most dehiscent cases were related to an earlier learning curve in the initial periods of glue-assisted surgery. First, we transplanted a graft of the same size on the bearing area, but this often resulted in a linear gap at the lateral border because of wound contraction or ocular horizontal movement. Later, we transplanted an oversized graft 1 mm larger at all borders than the size of the bearing area. Consequently, the dehiscence rate was greatly reduced from 44.4% (4 cases out of 9 total cases) in the first cases to 23.1% (3 cases out of 13 total cases) in the last cases (p = 0.276, Fisher's exact test). Although a small dehiscence of the lateral border appears clinically negligible, a larger gap or concurrent dehiscences in all 3 borders should be considered a cause for concern. In our study, 3 cases of pyogenic granuloma were observed in fibrin glue group and no cases of it in suturing group. This incomplete adaptation between the glued conjunctiva can activate fibroblasts or cause grafts to necrotize. This raises the possibility of increasing the risk of fibrosis in the surrounding graft or recurrence of the pterygium if the graft does not survive. The exact mechanism involved in the formation of pyogenic granuloma has not been completely elucidated, but associations with abnormal vascular endothelial cell growth, cytokine abnormalities, and fibroblasts activation have been postulated [29,30]. We believe that pyogenic granulomas in the present study were associated with a hyperreactive inflammatory change in the circumstance of abundant vascular endothelial cell growth factors originated from ischemic tissue damage. Therefore, we need to take caution not to make maladapted conjunctival wound margins when we use the glue. It can lead fibrosis or recurrence, causing the harms to outweigh the advantages of less inflammation in the glued conjunctiva.
The recurrence rate of pterygium, depending on the surgery type such as glue-assisted or suture-assisted autografting, has been a matter of controversy. Some reports [8,28] documented that the fibrin glue group showed lower recurrence rates than the suture group, whereas other reports [7] stated the converse. Hall et al. [23] reported similar recurrence rates for the two groups. In our study, the small number of cohort patients and a short follow-up period could not determine the long-term recurrence rate of glue-assisted pterygium surgery. Nevertheless, we believe that less inflammation in the earlier postoperative stage may be related to a small chance of recurrence; this hypothesis corresponds with the result of a previous report on correlation between inflammation and recurrence [4].
Our study had a limitation because of the retrospective approach, short postoperative follow-up period, and small cohort. In addition, the results could be affected by several biases such as the remaining sutures for anchoring graft for a month in the suture group, or differences in mean duration of follow up and age between the two groups. The learning curve of the surgeon is also considered as a significant limiting factor because all the surgeries using fibrin glue have been conducted after those using 10-0 nylon. Nonetheless, it is still noteworthy to report that glue-assisted pterygium surgery is an efficient procedure for Asian patients. A prospective and randomized clinical trial would be needed in the future.
In conclusion, glue-assisted pterygium surgery shortened the operation duration and reduced early postoperative inflammation as compared to suture-assisted pterygium surgery. The difference in complication rates between the two groups did not reach statistical significance, although dehiscence and pyogenic granulomas were observed more frequently in fibrin glue group. Glue-assisted pterygium surgery seems to be another option for the pterygium surgery considering its comparable clinical outcome to suture-assisted pterygium surgery, and the fact that it is associated with less discomfort due to a sutureless wound.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68:343–346. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakaishi H, Yamamoto M, Ishida M, et al. Pingueculae and pterygia in motorcycle policemen. Ind Health. 1997;35:325–329. doi: 10.2486/indhealth.35.325. [DOI] [PubMed] [Google Scholar]
- 3.Norn M, Franck C. Long-term changes in the outer part of the eye in welders. Prevalence of spheroid degeneration, pinguecula, pterygium, and corneal cicatrices. Acta Ophthalmol (Copenh) 1991;69:382–386. doi: 10.1111/j.1755-3768.1991.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 4.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 5.Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004;88:911–914. doi: 10.1136/bjo.2003.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uy HS, Reyes JM, Flores JD, Lim-Bon-Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005;112:667–671. doi: 10.1016/j.ophtha.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Bahar I, Weinberger D, Gaton DD, Avisar R. Fibrin glue versus vicryl sutures for primary conjunctival closure in pterygium surgery: long-term results. Curr Eye Res. 2007;32:399–405. doi: 10.1080/02713680701294723. [DOI] [PubMed] [Google Scholar]
- 8.Karalezli A, Kucukerdonmez C, Akova YA, et al. Fibrin glue versus sutures for conjunctival autografting in pterygium surgery: a prospective comparative study. Br J Ophthalmol. 2008;92:1206–1210. doi: 10.1136/bjo.2008.139600. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Dollin M, McAllum P, et al. Fibrin glue versus sutures for attaching the conjunctival autograft in pterygium surgery: a prospective observer masked clinical trial. Br J Ophthalmol. 2009;93:215–218. doi: 10.1136/bjo.2008.145516. [DOI] [PubMed] [Google Scholar]
- 10.Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115:1235–1240. doi: 10.1001/archopht.1997.01100160405001. [DOI] [PubMed] [Google Scholar]
- 11.Paris Fdos S, de Farias CC, Melo GB, et al. Postoperative subconjunctival corticosteroid injection to prevent pterygium recurrence. Cornea. 2008;27:406–410. doi: 10.1097/ICO.0b013e318162af90. [DOI] [PubMed] [Google Scholar]
- 12.Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007;18:308–313. doi: 10.1097/ICU.0b013e3281a7ecbb. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998;82:661–665. doi: 10.1136/bjo.82.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talu H, Tasindi E, Ciftci F, Yildiz TF. Excimer laser phototherapeutic keratectomy for recurrent pterygium. J Cataract Refract Surg. 1998;24:1326–1332. doi: 10.1016/s0886-3350(98)80223-x. [DOI] [PubMed] [Google Scholar]
- 15.Amano S, Motoyama Y, Oshika T, et al. Comparative study of intraoperative mitomycin C and beta irradiation in pterygium surgery. Br J Ophthalmol. 2000;84:618–621. doi: 10.1136/bjo.84.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaffrey CI, Spotnitz WD, Shaffrey ME, Jane JA. Neurosurgical applications of fibrin glue: augmentation of dural closure in 134 patients. Neurosurgery. 1990;26:207–210. [PubMed] [Google Scholar]
- 17.Gosain AK, Lyon VB Plastic Surgery Educational Foundation DATA Committee. The current status of tissue glues: part II. For adhesion of soft tissues. Plast Reconstr Surg. 2002;110:1581–1584. doi: 10.1097/01.PRS.0000033993.30838.3E. [DOI] [PubMed] [Google Scholar]
- 18.Bertrand B, Doyen A, Eloy P. Triosite implants and fibrin glue in the treatment of atrophic rhinitis: technique and results. Laryngoscope. 1996;106(5 Pt 1):652–657. doi: 10.1097/00005537-199605000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Dadeya S, Ms K. Strabismus surgery: fibrin glue versus vicryl for conjunctival closure. Acta Ophthalmol Scand. 2001;79:515–517. doi: 10.1034/j.1600-0420.2001.790518.x. [DOI] [PubMed] [Google Scholar]
- 20.Lagoutte FM, Gauthier L, Comte PR. A fibrin sealant for perforated and preperforated corneal ulcers. Br J Ophthalmol. 1989;73:757–761. doi: 10.1136/bjo.73.9.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hick S, Demers PE, Brunette I, et al. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24:369–377. doi: 10.1097/01.ico.0000151547.08113.d1. [DOI] [PubMed] [Google Scholar]
- 22.Ozdamar Y, Mutevelli S, Han U, et al. A comparative study of tissue glue and vicryl suture for closing limbal-conjunctival autografts and histologic evaluation after pterygium excision. Cornea. 2008;27:552–558. doi: 10.1097/ICO.0b013e318165b16d. [DOI] [PubMed] [Google Scholar]
- 23.Hall RC, Logan AJ, Wells AP. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin Experiment Ophthalmol. 2009;37:584–589. doi: 10.1111/j.1442-9071.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 24.Marticorena J, Rodriguez-Ares MT, Tourino R, et al. Pterygium surgery: conjunctival autograft using a fibrin adhesive. Cornea. 2006;25:34–36. doi: 10.1097/01.ico.0000164780.25914.0a. [DOI] [PubMed] [Google Scholar]
- 25.Alvarenga LS. Comments on using fibrin glue in pterygium surgery. Br J Ophthalmol. 2005;89:392. doi: 10.1136/bjo.2004.054031/10.1136/bjo.2004.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan SM, Boisjoly H. Advances in the use of adhesives in ophthalmology. Curr Opin Ophthalmol. 2004;15:305–310. doi: 10.1097/00055735-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hino M, Ishiko O, Honda KI, et al. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108:194–195. doi: 10.1046/j.1365-2141.2000.01818.x. [DOI] [PubMed] [Google Scholar]
- 28.Koranyi G, Seregard S, Kopp ED. The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 2005;83:298–301. doi: 10.1111/j.1600-0420.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 29.Freitas TM, Miguel MC, Silveira EJ, et al. Assessment of angiogenic markers in oral hemangiomas and pyogenic granulomas. Exp Mol Pathol. 2005;79:79–85. doi: 10.1016/j.yexmp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Yuan K, Wing LY, Lin MT. Pathogenetic roles of angiogenic factors in pyogenic granulomas in pregnancy are modulated by female sex hormones. J Periodontol. 2002;73:701–708. doi: 10.1902/jop.2002.73.7.701. [DOI] [PubMed] [Google Scholar]