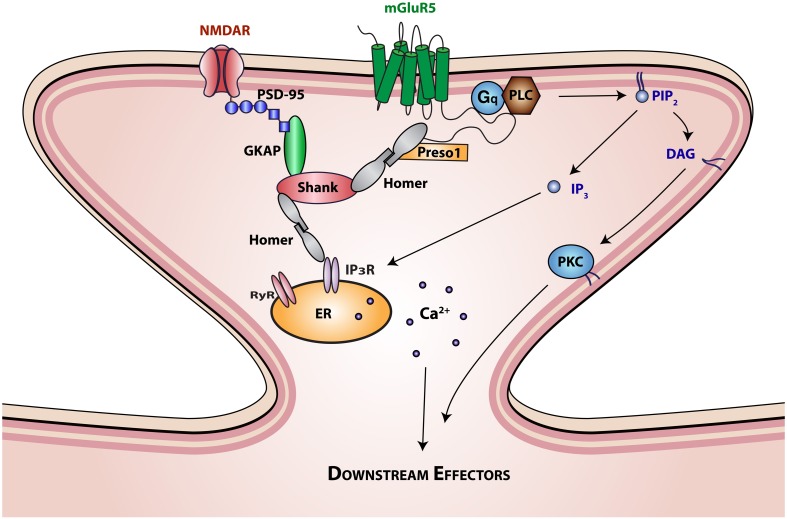
Figure 1.
Interaction of mGluR5 with scaffolding proteins and signaling molecules. The SH3-multiple ankyrin domain-containing protein (Shank) is a prototypical PDZ scaffolding protein. The PDZ domain of Shank interacts with the c terminus of guanylate kinase-associated protein (GKAP), which is in turn associated with the ionotropic glutamatergic N-methyl-D-aspartate (NMDA) receptor-PSD95 complex. The proline rich domain of Shank interacts with the EVH domain of Homer proteins. Homer proteins form multimers through interactions of their coiled-coil domain and link Shank to mGluR5 and inositol triphosphate (IP3) or ryanodine receptors. Homer interactions with mGluR5 are further regulated by Preso1 scaffolding proteins. mGluR5 activation by glutamate initiates Gq protein signaling that regulates the function of phospholipase C (PLC). Activation of PLC results in the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to release the second messengers 1,2-Diacylglycerol (DAG) and IP3. DAG is the physiological activator of protein kinase C (PKC), which in turn activates various intracellular signaling cascades. IP3 binds to intracellular IP3 receptors (IP3R) on the endoplasmic reticulum (ER) membrane initiating Ca2+ release from the ER lumen into the cytoplasm, generating complex Ca2+ concentration-dependent signals, including temporal oscillations, and propagating waves.