Summary
Our previous study demonstrated that tyrosine phosphorylation of p145met/β-subunit of hepatocyte growth factor receptor by epidermal growth factor receptor and Src contributes to the anti-apoptotic growth of human bladder carcinoma cell 5637 under serum-starved conditions. Here, we show that some other cell lines of human bladder carcinoma, but not other types of human cancer cells, also exhibit Src-dependent, anti-apoptotic proliferation under serum-starved conditions, and that low-density, detergent-insoluble membrane microdomains (MD) serve as a structural platform for signaling events involving p145met, EGFR, and Src. As an MD-associated molecule that may contribute to bladder carcinoma-specific cellular function, we identified uroplakin IIIa (UPIIIa), an urothelium-specific protein. Results obtained so far revealed: 1) UPIIIa undergoes partial proteolysis in serum-starved cells; 2) a specific antibody to the extracellular domain of UPIIIa inhibits the proteolysis of UPIIIa and the activation of Src, and promotes apoptosis in serum-starved cells; and 3) knockdown of UPIIIa by short interfering RNA also promotes apoptosis in serum-starved cells. GM6001, a potent inhibitor of matrix metalloproteinase (MMP), inhibits the proteolysis of UPIIIa and promotes apoptosis in serum-starved cells. Furthermore, serum starvation promotes expression and secretion of the heparin-binding EGF-like growth factor in a manner that depends on the functions of MMP, Src, and UPIIIa. These results highlight a hitherto unknown signaling network involving a subset of MD-associated molecules in the anti-apoptotic mechanisms of human bladder carcinoma cells.
Key words: Anti-apoptosis, Bladder cancer, Membrane microdomains, Src, Uroplakin III
Introduction
Cancer cells acquire one or more of the cancer cell-specific functions upon establishment of their malignancy. They include self-sufficiency of proliferation, insensitivity to anti-proliferation signals, sustained angiogenesis, anti-apoptotic growth (as highlighted in this study), limitless replication of genomic DNA, and tissue invasion and metastasis (Hanahan and Weinberg, 2000). Since the initial discovery of Ras protein as a causative gene product for some cases of bladder carcinoma in human, much attention has been paid to understand the relationship between cellular signal transduction and human cancer (Hanahan and Weinberg, 2000). Src and epidermal growth factor receptor/kinase (EGFR/kinase) are also classically as well as contemporary well-known examples, whose de-regulated protein functions by virtue of genetic mutations and/or altered expression level lead to an initiation, promotion, and progression of malignant cell transformation. Involvement of Src and/or EGFR/kinase in a number of specific cases of human cancer has become well documented and many specific inhibitors to them are now in use for clinical settings or trials (Aleshin and Finn, 2010; Hunter, 2009; Hynes and Lane, 2005). Against this background, however, the role played by Src and/or EGFR/kinase in bladder cancer is not well understood until recently (see below).
When a population of cancer cells arises in the vicinity of normal cells and tissues, there will be an insufficiency or complete lack of blood supply to those cancer cells. A similar type of shortage could also occur for cancer cells at later stages (e.g. upon invasive propagation). These conditions, involving lowered levels of oxygen pressure (i.e. hypoxia) and other factors for survival (e.g. growth factors and other nutrient compounds, as highlighted in this study), would act as selective pressure for cancer cells, by which only those successfully adapted would continue their malignant growth. It is well known that cancer cells overcome these kinds of environmental pressures by promoting several responses to hypoxia that include altered gene expression (e.g. up-regulation of hypoxia-inducible genes) and signal transduction for angiogenesis (e.g. expression of vascular endothelial growth factor) (Griffin and Shockcor, 2004; Harris, 2002). On the other hand, the molecular mechanisms of cancer cell adaptation to a shortage of survival factors remain less clear (for a review, see Pirkmajer and Chibalin, 2011).
Protein-tyrosine phosphorylation is a well-known mechanism of intracellular signal transduction, by which the transmembrane growth-controlling events (e.g. the binding of growth factor to the cognate cell surface receptor) are translated to the activation of cytoplasmic protein functions and nuclear gene expression. In general, protein-tyrosine phosphorylation catalyzed by receptor/kinase (e.g. EGFR/kinase) and/or cytoplasmic kinase (e.g. Src and other Src family kinases) in the normal cell system is a rapid and transient phenomenon in response to a transmembrane growth-promoting signal(s) (Hunter, 2009; Thomas and Brugge, 1997). In previous studies using 5637 human bladder carcinoma cells, serum-independent growth was reported (Ruck et al., 1994; Ruck and Paulie, 1997; Ruck and Paulie, 1998); however, we found that the activity of the tyrosine kinase Src became stably up-regulated after several hours of serum deprivation in the culture media (Yamamoto et al., 2006). Further studies demonstrated that the activated Src contributed to the anti-apoptotic growth of the 5637 cells under serum-starved conditions through phosphorylation of the β-subunit of c-Met/hepatocyte growth factor (HGF) receptor. In the present study, we have tried to achieve further insight into the anti-apoptotic mechanism involving Src by focusing on the importance of cholesterol-enriched membrane microdomains (MDs) and their associated molecules, whose architecture and cellular functions have recently been extensively studied (Brown and London, 1998; Hemler, 2005; Mahbub Hasan et al., 2011; Simons and Toomre, 2000). We demonstrate that MD-associated uroplakin IIIa, a transmembrane protein that has been originally identified and characterized as a major component of asymmetric unit membrane (AUM) of urinary bladder epithelium (Jenkins and Woolf, 2007; Khandelwal et al., 2009; Wu et al., 2009; Wu et al., 1994; Wu and Sun, 1993), plays an important role in the Src-dependent signal transduction.
Materials and Methods
Antibodies, inhibitors, and other reagents
Mouse anti-phosphotyrosine antibody (PY99) and rabbit anti-Met/HGF receptor antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-Src antibody was obtained from Oncogene Research Products (San Diego, CA). Rabbit anti-active Src (phosphotyrosine-418 or pY418) and anti-inactive Src (phosphotyrosine-527 or pY529) antibodies were obtained from Biosource International (Camarillo, CA). Rabbit anti-active mitogen-activated protein kinase (phospho-MAPK or pMAPK) was from Cell Signaling Technology (Danvers, MA). Mouse antibody against β-tubulin was from BD Biosciences (Tokyo, Japan). Rabbit antibodies to the carboxyl-terminal region of human EGFR (anti-EGFR antibody), the extracellular domain or the carboxyl-terminal region of Xenopus laevis uroplakin III (UPIII) (anti-UPIII-ED and anti-UPIII-CT antibodies) were prepared as previously described (Mahbub Hasan et al., 2005; Sakakibara et al., 2005; Sato et al., 1995). Rabbit anti-human UPIIIa antibody was raised against a synthetic peptide that corresponds to residues 274–287 of the human UPIIIa (PLDRAEVYSSKLQD). Mouse anti-EGF antibody (mAb 528), goat anti-heparin binding EGF-like growth factor (HB-EGF) antibody (Ab-1), Src-specific tyrosine kinase inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), its inactive analogs PP3 (4-amino-7-phenylpyrazol[3,4-d]pyrimidine), a broad-specificity inhibitor of matrix metalloproteinase (MMP) (N-[(2R)-2-(hydroxamido-carbonylmethyl)-4- methylpentanoyl]-L-tryptophan methylamide, GM6001), and its inactive analog GM6001 n.c. (N-t-butoxycarbonyl-L-leucyl-L-tryptophan methylamide), and a specific inhibitor for MAPK kinase (MEK) U0126 were obtained from Calbiochem-EMD Chemicals USA (Gibbstown, NJ). Protease inhibitors, leupeptin and (p-amidinophenyl)methane-sulfonyl fluoride hydrochloride (APMSF), were purchased from the Peptide Institute (Osaka, Japan) and Wako Pure Chemicals, respectively. Methyl-β-cyclodextrin was from Wako Pure Chemicals (Osaka, Japan). A synthetic substrate for caspase 3/7, Ac-Asp-Glu-Val-Asp-AMC (Ac-DEVD-AMC), was obtained from Calbiochem-EMD Chemicals USA. Protein A-Sepharose was obtained from Amersham Biosciences (Uppsala, Sweden). Other chemicals were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO), Wako Pure Chemicals, or Nacalai Tesque (Kyoto, Japan).
Cell culture and transfection
The following human cell lines were obtained from American Type Culture Collection (Rockville, MD): bladder carcinoma cell lines 5637, HT-1197, HT-1376, and UMUC-3, kidney carcinoma cell line ACHN, colon carcinoma cell line HT-29, breast cancer cell line MCF7, epidermoid carcinoma cell line A431, and embryonic kidney cell line 293; and were maintained in either DMEM or RPMI1640 medium supplemented with 10% fetal calf serum (FCS), 1 mM L-glutamine, and antibiotics. Cells were grown at 37°C in a humidified 5% CO2 atmosphere. For all experiments, confluent cells were trypsinized, and aliquots of single-cell suspension were re-seeded and cultured in poly-L-lysine-coated new culture dishes containing normal culture medium for 24–48 h. After the normal growth treatment, cells were treated in several different conditions as specified in the text. Quantification of cell number and cell viability was carried out as described previously (Yamamoto et al., 2006). For exogenous expression of FLAG-tagged kinase-negative or wild type Src, cells at 40–60% confluence in 60-mm dishes were incubated with 6 µg plasmid DNA per dish for 24 h using FuGeneTM reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocol. For knockdown of UPIIIa, cells at 20–40% confluence in 35-mm dishes were transfected with none (DEPC-treated water alone), control short interference RNA (siRNA) (scrambled), or siRNA directed against UPIIIa, for 24–36 h using TransIT-siTKO reagents (MirusCorp., Madison, WI) according to the manufacturer's instructions. Three different silencer siRNA oligos for UPIIIa, whose sequences were not disclosed by the vendor, were purchased from Invitrogen (Carlsbad, CA). After the transfection treatments described above, cells were cultured under different conditions as specified in the text, analyzed for their proliferation and viability as described above, and processed for extraction and fractionation as described below.
Cell extraction and fractionation
All manipulations were carried out on ice or at 4°C. Cells in 60-mm dishes were washed twice with ice-cold phosphate-buffered saline (PBS) and supplemented with 0.15–0.3 ml of buffer per dish containing 1% Triton X-100, 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 10 mM β-mercaptoethanol, 1 mM sodium orthovanadate, 10 µg/ml leupeptin, and 20 µM APMSF. The mixtures were collected and homogenized with a Dounce homogenizer by 10–15 strokes, followed by incubation on ice for 10 min. The cell homogenates were then subjected to centrifugation at 500 g for 10 min to remove debris, and the supernatants were further centrifuged at 150,000 g for 20 min. The resulting supernatants were collected as Triton X-100-solubilized whole cell lysates (WCLs) and used for experiments. Alternatively, when low-density, detergent-insoluble (cholesterol-enriched) MDs were required, the debris-free supernatants prepared as above were subjected to discontinuous sucrose density gradient ultracentrifugation as described previously (Mahbub Hasan et al., 2007).
SDS-PAGE, immunoprecipitation, and immunoblotting
Protein samples, the amounts as specified in the text, were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels as previously described (Laemmli, 1970). Immunoprecipitation and immunoblotting using specific antibodies as specified in the text were performed as described previously (Yamamoto et al., 2006).
Enzyme-linked immunosorbent assay (ELISA)
The amounts of EGF and HB-EGF released into culture media from 5637 cells were quantified by ELISA. The culture media at the specified times of cell culture were collected and centrifuged in CentriconTM tubes (Millipore, Billerica, MA) at 3,000 g for 10–30 min. The concentrated supernatants were applied to a 96-well plate overnight at 4°C. After the overnight incubation, wells were blocked with BlockAce (Dainihon-Seiyaku, Japan) overnight at 4°C. Wells were then washed three times (3 min per wash) in PBS supplemented with 0.05% Tween 20 (T-PBS). Wells were then supplemented with the anti-EGF antibody, the anti-HB-EGF antibody, or the diluents for the antibodies (PBS containing 0.1% bovine serum albumin, B-PBS) alone. The antibody treatment proceeded for 2–3 h at room temperature and was terminated by washing with T-PBS (3 min × 3 times). Horseradish peroxidase-conjugated anti-mouse IgG or anti-goat IgG (Cappel, Germany) was then applied to wells at a dilution of 1:5,000 in B-PBS. The secondary antibody incubation proceeded for 2–3 h at room temperature and was terminated by washing with T-PBS (3 min × 3 times). The binding of the antibodies was demonstrated by the addition of a developing solution containing 66 mM NaHPO4, 33 mM citric acid, 0.015% H2O2, and 1 mg/mL O-phenylenediamine dichloride (OPD) compound (Sigma) for 20 min at room temperature. The color development was terminated by the addition of 2N H2SO4. Colorimetric determination was carried out with a spectrophotometer set at 492 nm.
mRNA analysis
Total RNAs were extracted from 5637 cells using an RNeasy Plus Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The following manipulations and analyses were performed by Bio Matrix Research Inc. (Nagareyama, Japan). Quantity and/or quality of RNA samples were verified using a spectrophotometer and an electropherogram. To evaluate the expression level of mRNA for HB-EGF and EGF, and all other genes, the RNA samples were then analyzed using a microarray platform, GeneChip®Human Genome U133 PLUS 2.0 Array. Data obtained were analyzed using the GeneSpring® program.
Other methods and statistics
Protein content in the samples was determined spectrophotometrically using an assay kit (Nacalai Tesque). Enzyme assays for caspase 3/7 in the whole cell extracts were carried out as described previously (Yamamoto et al., 2006). All immunoblotting data are representative of more than three independent experiments. Bars in the graphical data are means ± standard deviations of three independent experiments.
Results
Bladder carcinoma-specific, Src-dependent, and MAPK-independent proliferation under serum-starved culture conditions
We initially examined whether the Src-dependent survival and growth could be generally seen in serum-starved human bladder carcinomas (malignancy grade of 3–4) other than 5637 cells (malignancy grade of 2). Serum starvation promotes activation of Src in 5637 cells (2.2±0.2 fold, n = 4), as determined by the elevation of phosphorylation of tyrosine-418 in the Src molecule, and in two other cell lines: HT1197 (malignancy grade of 4) (1.4±0.3 fold, n = 4) and HT1376 (malignancy grade of 3) (2.8±0.6 fold, n = 4) (Fig. 1A). HT1197 and HT1376, like 5637 cells, exhibit serum-independent proliferation that could be blocked by PP2, an Src-specific inhibitor (Fig. 1B; supplementary material Fig. S1). In these cell lines, tyrosine phosphorylation of some other cellular proteins is also stimulated (data not shown). Under the same conditions, however, MAPK, a well-known protein kinase that is activated in response to serum stimulation (supplementary material Fig. S2), is not activated (Fig. 1A), as judged by the elevation of activating phosphorylation in normalized amounts of 42- and 44-kDa MAPK (data not shown). In addition, U0126, a potent inhibitor of the MAPK kinase (MEK), does not inhibit the serum-independent proliferation (Fig. 1B). On the other hand, UMUC3, another cell line of human bladder carcinoma (malignancy grade of 3), does not show any sign of Src activation (0.8±0.3 fold, n = 4) or proliferation under serum-starved conditions (Fig. 1A,B; supplementary material Fig. S1). It should also be noted that the expression level of Src in UMUC3 cells is much lower than that in other bladder carcinomas (Fig. 1A). Other types of human cancer or immortalized cells (renal carcinoma ACHN, 1.2±0.4-fold, n = 3; colon carcinoma HT29, 1.0±0.1-fold, n = 3; epidermoid carcinoma A431, 0.7±0.1-fold, n = 3; breast adenocarcinoma MCF7, 0.6±0.4-fold, n = 3; and embryonic kidney cell 293, 1.3±0.5-fold, n = 4) do not show any sign of Src activation under serum-starved conditions (Fig. 1). Interestingly, ACHN and A431 cells exhibit serum-independent and PP2-sensitive proliferation (Fig. 1B; supplementary material Fig. S1). However, proliferation of these cells is also sensitive to U0126 (Fig. 1B). There is a possibility that serum-independent proliferation of ACHN and A431 cells require basal activity of both Src and MAPK. Taking these findings together, we conclude that only a certain kind of bladder carcinoma cell is capable of proliferating under serum-starved conditions in an Src-dependent and MAPK-independent manner.
Fig. 1. Src, but not MAPK, contributes to the survival and proliferation of human bladder carcinoma cells in serum-starved conditions.
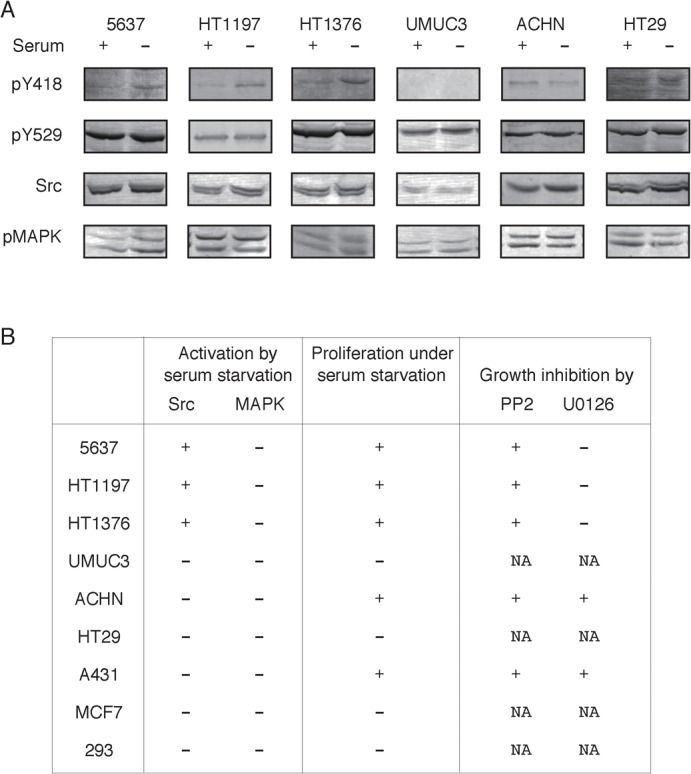
(A) Six carcinoma cells of human origin (bladder: 5637, HT1197, HT1376, and UMUC3; kidney: ACHN; colon: HT29) were cultured under normal, serum-containing (serum +) or serum-starved conditions (serum −) for 24 h. After the treatments, the cells were extracted with Triton X-100-containing buffer and subjected to SDS-PAGE and immunoblotting with the indicated antibodies (phosphotyrosine-418 of Src, phosphotyrosine-529 of Src, Src, and phospho-MAPK) as described in Materials and Methods. (B) Human carcinoma cells used in panel A and some other human carcinoma or immortalized cell lines (A431, MCF7, and 293) (0.1×106 cells/dish) were analyzed for the occurrence of activation of Src and MAPK. Symbols + and − denote the occurrence of activation of Src and MAPK (for detail, see main text). Note that only statistically significant values were taken as those showing “Src or MAPK is activated”. Occurrence of active proliferation under serum-starved culture conditions, and its sensitivity to either PP2 or U0126 was also examined as described in Materials and Methods and data are presented by symbols + or −. N.A., not applicable (in the case of cells showing no active proliferation in response to serum starvation).
Requirement of Src activity for survival and proliferation in 5637 cells under serum-starved culture conditions
To evaluate further the specific importance of Src in serum-independent proliferation, 5637 cells expressing FLAG-tagged Src of either kinase-negative mutant (KN-Src) or wild type (WT-Src) and control cells were cultured in normal or serum-deprived conditions, and analyzed for their biochemical and cell biological properties. In control cells, serum starvation promoted tyrosine phosphorylation of p145met and endogenous Src, indicative of Src activation (Fig. 2A, lanes 1, 4). Serum-starved control cells exhibited an active proliferation (Fig. 2B) and a very low incidence in cell death (Fig. 2C), as seen in the serum-added control cells (Yamamoto et al., 2006). However, cells expressing KN-Src did not promote tyrosine phosphorylation in response to serum starvation (Fig. 2A, lanes 2, 5), and showed a reduced proliferation rate and signs of apoptosis, namely, elevated cell death and activation of caspase 3/7 (Fig. 2B,C). On the other hand, cells expressing WT-Src promoted tyrosine phosphorylation irrespective of culture conditions (Fig. 2A, lanes 3, 6), and showed a similar viability to the control cells (Fig. 2B,C). It should also be noted that, under serum-containing culture conditions, cell expressing KN-Src exhibited an active proliferation as seen in cells expressing WT-Src or control cells (data not shown). These results demonstrate that inhibition of Src activity impairs the ability of 5637 cells to survive and proliferate under serum-deprived culture conditions.
Fig. 2. Src activity is required for survival and growth of 5637 cells in serum-free conditions.
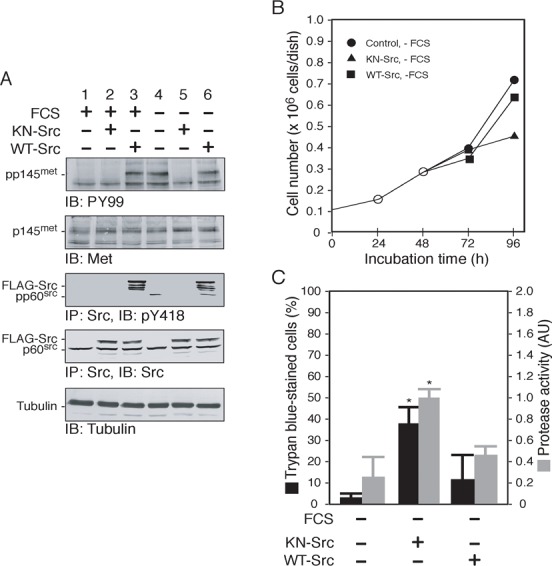
(A) 5637 cells were transfected with a control plasmid or plasmid expressing FLAG-tagged kinase-negative Src (KN-Src) or FLAG-tagged wild-type Src (WT-Src) as described in Materials and Methods. After the transfection, cells were cultured in either serum-containing medium (FCS +) or serum-free medium (FCS −) for 24 h. Triton X-100-solubilized cell extracts were prepared and analyzed by immunoprecipitation (IP, 300 µg/lane) and/or immunoblotting (IB, 30 µg/lane) with the indicated antibodies as described in Materials and Methods. The positions of the tyrosine-phosphorylated forms or the total proteins of p145met, FLAG-tagged or endogenous Src, and tubulin are indicated. (B) 5637 cells (0.1×106 cells/dish) were cultured in serum-containing conditions for 24 h (○), transfected with a control plasmid ( ), KN-Src (▴), or WT-Src (▪) for 24 h as in panel A, and then cultured in serum-containing (FCS +) or serum-free medium (FCS −) for 48 h. Cell number was determined at 24, 48, 72 and 96 hours of incubation. (C) 5637 cells were treated as in panel B. After the treatments (96 h post incubation), cell death (black bars, shown as percentage of total cells) and caspase 3/7 protease activity of Triton X-100-solubilized cell extracts (20 µg/assay) (grey bars, shown as arbitrary unit) were determined by Trypan Blue exclusion and a synthetic substrate Ac-DEVD-AMC, respectively, as described in Materials and Methods. Data shown are mean ± s.d. of three independent experiments. *P<0.01 compared with control.
), KN-Src (▴), or WT-Src (▪) for 24 h as in panel A, and then cultured in serum-containing (FCS +) or serum-free medium (FCS −) for 48 h. Cell number was determined at 24, 48, 72 and 96 hours of incubation. (C) 5637 cells were treated as in panel B. After the treatments (96 h post incubation), cell death (black bars, shown as percentage of total cells) and caspase 3/7 protease activity of Triton X-100-solubilized cell extracts (20 µg/assay) (grey bars, shown as arbitrary unit) were determined by Trypan Blue exclusion and a synthetic substrate Ac-DEVD-AMC, respectively, as described in Materials and Methods. Data shown are mean ± s.d. of three independent experiments. *P<0.01 compared with control.
Cholesterol-enriched MDs play a role in the anti-apoptosis of serum-starved 5637 cells
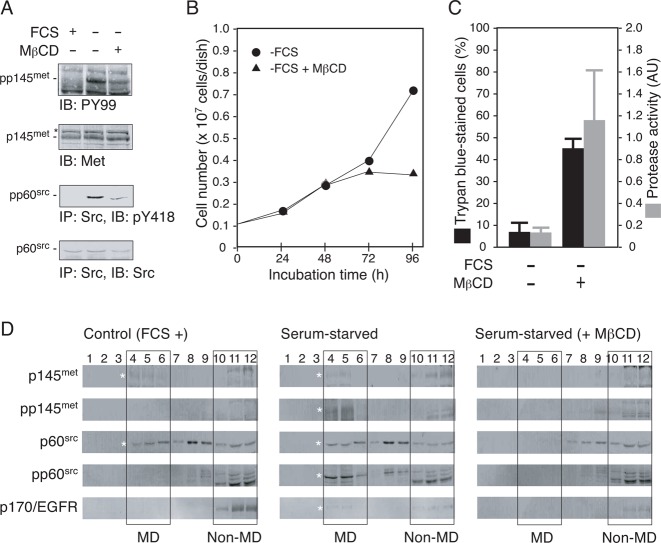
We next examined the effect of methyl-β-cyclodextrin (MβCD), a substance that disrupts the cholesterol-dependent membrane structures, on the anti-apoptotic signal transduction and growth of serum-starved 5637 cells. Immunoblotting experiments demonstrated that MβCD inhibited the tyrosine phosphorylation of p145met and Src in serum-starved 5637 cells (Fig. 3A). The MβCD treatment blocked cell proliferation and induced cell death accompanied by stimulation of the caspase 3/7 activity (Fig. 3B,C). In normally cultured 5637 cells, a fraction of both p145met and Src, but not EGFR, localizes to the low-density, detergent-insoluble membrane (i.e. MD) fractions (Fig. 3D, control, FCS +). In serum-starved cells, tyrosine phosphorylated forms of p145met and Src predominantly localized to the MD fractions. Under the same conditions, EGFR was also recruited to the MD fractions (Fig. 3D, serum-starved). Co-incubation of serum-starved 5637 cells with MβCD, however, disrupted all of these characteristics (Fig. 3D, + MβCD).
Fig. 3. Disruption of cholesterol-dependent MDs interferes with Src-dependent signal transduction and promotes apoptosis in serum-starved 5637 cells.
(A) 5637 cells were grown in serum-free condition in the absence or the presence of methyl-β-cyclodextrin (MβCD, 15 µM) for 8 h. After the treatments, Triton X-100-solubilized cell extracts were prepared and analyzed for tyrosine phosphorylation of p145met and p60src by immunoprecipitation (300 µg/lane) and/or immunoblotting (30 µg/lane). Control data obtained with the extracts prepared from cells grown in serum-containing medium (FCS +) are also shown. (B) 5637 cells (0.1×107 cells/dish) were grown in serum-containing condition for 48 h and then exposed to serum-free medium with (▴) or without ( ) 15 µM MβCD for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 2B. (C) 5637 cells were cultured in serum-containing (FCS +) or serum-free (FCS −) medium with or without 15 µM MβCD as in panel B for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. (D) 5637 cells were cultured in serum-containing medium (control, FCS +), serum-free medium in the absence (serum-starved) or the presence of 15 µM MβCD (serum-starved, + MβCD). Cell samples were subjected to subcellular fractionation by discontinuous sucrose density gradient ultracentrifugation as described in Materials and Methods. Twelve fractions obtained were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Rectangles indicate the positions of low density, detergent-insoluble MD fractions (fractions 4–6, denoted as “MD”) and non-MD fractions (fractions 10–12, denoted as “non-MD”). Asterisks indicate the positions of proteins of interest.
) 15 µM MβCD for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 2B. (C) 5637 cells were cultured in serum-containing (FCS +) or serum-free (FCS −) medium with or without 15 µM MβCD as in panel B for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. (D) 5637 cells were cultured in serum-containing medium (control, FCS +), serum-free medium in the absence (serum-starved) or the presence of 15 µM MβCD (serum-starved, + MβCD). Cell samples were subjected to subcellular fractionation by discontinuous sucrose density gradient ultracentrifugation as described in Materials and Methods. Twelve fractions obtained were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Rectangles indicate the positions of low density, detergent-insoluble MD fractions (fractions 4–6, denoted as “MD”) and non-MD fractions (fractions 10–12, denoted as “non-MD”). Asterisks indicate the positions of proteins of interest.
MD-associated protein uroplakin III undergoes partial proteolysis in serum-starved 5637 cells
Results obtained from the study on MD led us to analyze the expression, localization, and function of a single-transmembrane protein, UPIIIa. In our previous studies on signal transduction of fertilization in Xenopus laevis, African clawed frog, UPIIIa and Src were shown to interact with each other to accomplish gamete interaction and subsequent induction of embryonic development (for a review, see Mahbub Hasan et al., 2011). UPIIIa was originally identified as an urothelial tissue-specific structural protein, and thus should make an important contribution to bladder-specific cancer cell functions. Immunoblotting experiments demonstrated that an antibody against the extracellular domain of Xenopus laevis UPIII (anti-UPIII-ED antibody) recognized a protein of 45 kDa, which is similar to known molecular sizes of mammalian UPIIIa protein on SDS-PAGE gels, in 5637 cells (Fig. 4A; supplementary material Fig. S3). The 45-kDa protein was also recognized by another antibody that was raised against the carboxyl-terminal sequence of human UPIIIa (data not shown). In addition, recombinant protein of the extracellular domain of Xenopus laevis UPIII interferes with the recognition of the 45-kDa protein, and treatment of the 5637 cell extracts with N-glycosidase resulted in a downshift of the 45-kDa band's mobility to 30 kDa, a known feature of mammalian UPIII (data not shown). From these data, we concluded that the immunoreactive 45-kDa protein is UPIIIa and conducted further experiments.
Fig. 4. Serum starvation promotes partial proteolysis of UPIIIa in 5637 cells.
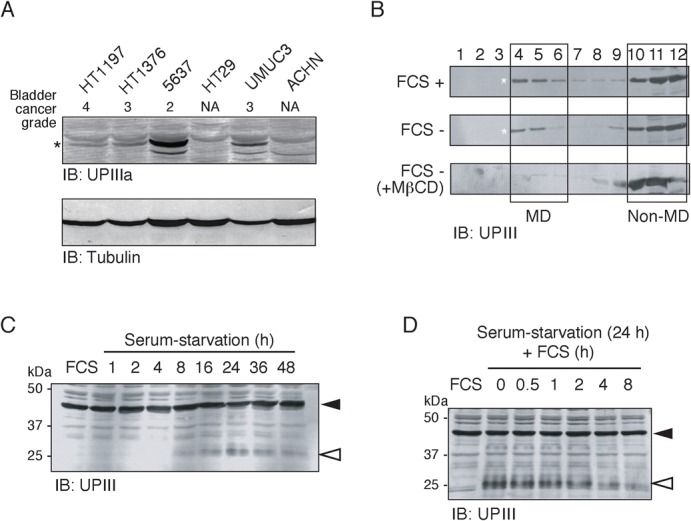
(A) Expression level of UPIIIa in 5637 and some other human cancer cell lines. (B) 5637 cells were cultured in serum-containing medium (FCS +), serum-free medium in the absence (FCS −) or the presence of 15 µM MβCD (FCS −, MβCD). Cell samples were subjected to subcellular fractionation by discontinuous sucrose density gradient ultracentrifugation as in Fig. 3D, and fractions obtained were analyzed by immunoblotting with the anti-UPIII antibody. The positions of the MD and the non-MD fractions were indicated by opened squares. Asterisks indicate the positions of the 45-kDa UPIIIa. (C) Triton X-100-solubilized cell extracts were prepared from 5637 cells that had been exposed to serum-free medium for the indicated times (0–48 h). Protein samples (30 µg/lane) were analyzed by immunoblotting with anti-UPIII antibody. Closed and opened arrowheads indicate the positions of the 45-kDa UPIIIa and its degraded fragment, respectively. (D) Serum-starved 5637 cells (24 h of serum starvation) were treated with serum-containing medium for the indicated times (0–8 h). Closed and opened arrowheads indicate the positions of the 45-kDa UPIIIa and its degraded fragment, respectively. In panels C and D, control data obtained with the normally-cultured 5637 cells (FCS) are also shown.
We next examined the expression of UPIIIa in other cell lines including non-bladder carcinomas. Immunoblotting experiments demonstrated that UPIIIa was expressed in all of the four bladder carcinomas, but not in non-bladder carcinomas (Fig. 4A). In bladder carcinomas, the expression level of UPIIIa, as normalized by the protein amounts tested (Fig. 4A, tubulin data), was higher in 5637, intermediate in UMUC3, and lower in HT1197 and HT1376, showing an inverse correlation with the malignancy grade of bladder cancer.
Subcellular fractionation studies demonstrated that UPIIIa localizes to both MD and non-MD fractions before and after serum starvation (Fig. 4B). In MβCD-treated 5637 cells, the localization of UPIIIa to the MDs was, like that of p145met, Src, and EGFR, entirely disrupted in both control (data not shown) and serum-starved culture conditions (Fig. 3B). Unlike p145met, Src, and EGFR, however, UPIIIa is not phosphorylated on tyrosine residues in response to serum starvation (data not shown). On the other hand, immunoblotting experiments demonstrated that UPIIIa underwent partial proteolysis, in which anti-UPIII immunoreactive bands of ∼25-kDa protein were newly appeared, in the course of serum starvation (Fig. 4C) that could be reversed by the addition of serum (Fig. 4D).
Uroplakin IIIa and matrix metalloproteinase play important roles in the anti-apoptosis of serum-starved 5637 cells
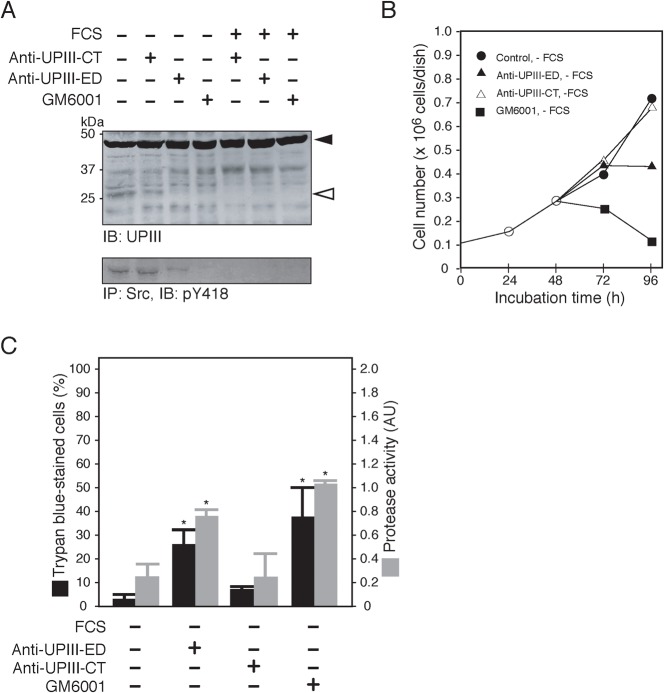
We then tried to explore the potential role of partial proteolysis of UPIIIa in serum-starved 5637 cells. To this end, we first surveyed a substance that could block the proteolysis of UPIIIa. In Xenopus laevis, it has been shown that two synthetic peptides that contain an Arg-Arg sequence, namely, UPIII-GRR peptide and BOC-GRR-MCA peptide (Mahbub Hasan et al., 2005), are inhibitory to partial proteolysis of UPIII by sperm-derived tryptic protease or by the purified cathepsin B. However, these peptides did not show any effect on the proteolysis of UPIIIa in serum-starved 5637 cells (supplementary material Fig. S4). On the other hand, the anti-UPIII-ED antibody and GM6001, a matrix metalloproteinase-specific inhibitor, were shown to inhibit the proteolysis of UPIIIa (Fig. 5A). Under the same conditions, tyrosine phosphorylation of Src was also inhibited (Fig. 5A), and the cells showed a reduced rate of proliferation (Fig. 5B) and underwent apoptotic cell death (Fig. 5C). These results suggest that partial proteolysis of UPIIIa is required for the activation of Src in serum-starved 5637 cells. However, an Src-specific inhibitor, PP2, which was shown to inhibit the activation of Src and the survival of 5637 cells (Yamamoto et al., 2006), also inhibited the proteolysis of UPIIIa (supplementary material Fig. S5), indicating that the activation of Src and the proteolysis of UPIIIa depend on each other (see Discussion).
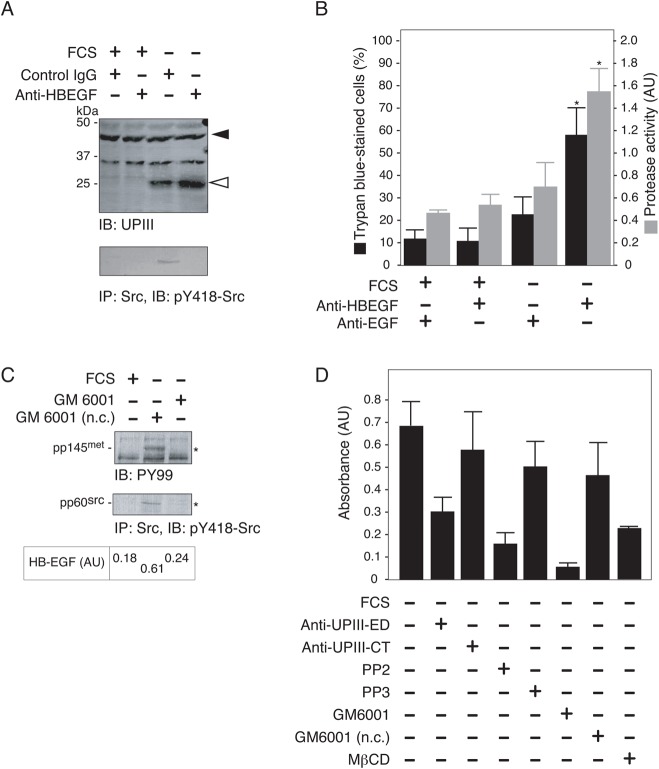
Fig. 5. GM6001 and anti-UPIII antibody inhibit Src-dependent signal transduction and proteolysis of UPIII, and promote apoptosis in serum-starved 5637 cells.
(A) 5637 cells were cultured in serum-free (FCS −) or serum-containing (FCS +) conditions for 24 h in the absence or the presence of 100 µg/ml anti-UPIII-CT IgG (CT), 100 µg/ml anti-UPIII-ED IgG (ED), or 10 µM GM6001. Partial proteolysis of UPIII (IB: UPIII), and tyrosine phosphorylation of Src (IP: Src, IB: pY418) were analyzed by immunoblotting of the Triton X-100-solubilized cell extracts (20 µg/lane). (B) 5637 cells (0.1×106 cells/dish) were grown in serum-containing condition for 48 h and then exposed to serum-free medium in the absence ( ) or the presence of 100 µg/ml anti-UPIII-ED antibody (▴), 100 µg/ml anti-UPIII-CT antibody (▵), or 10 µM GM6001 (▪) for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 1B. (C) 5637 cells were cultured in serum-free medium (FCS −) in the absence or the presence of 100 µg/ml anti-UPIII-ED antibody, 100 µg/ml anti-UPIII-CT antibody, or 10 µM GM6001 for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. *P<0.01 compared with control.
) or the presence of 100 µg/ml anti-UPIII-ED antibody (▴), 100 µg/ml anti-UPIII-CT antibody (▵), or 10 µM GM6001 (▪) for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 1B. (C) 5637 cells were cultured in serum-free medium (FCS −) in the absence or the presence of 100 µg/ml anti-UPIII-ED antibody, 100 µg/ml anti-UPIII-CT antibody, or 10 µM GM6001 for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. *P<0.01 compared with control.
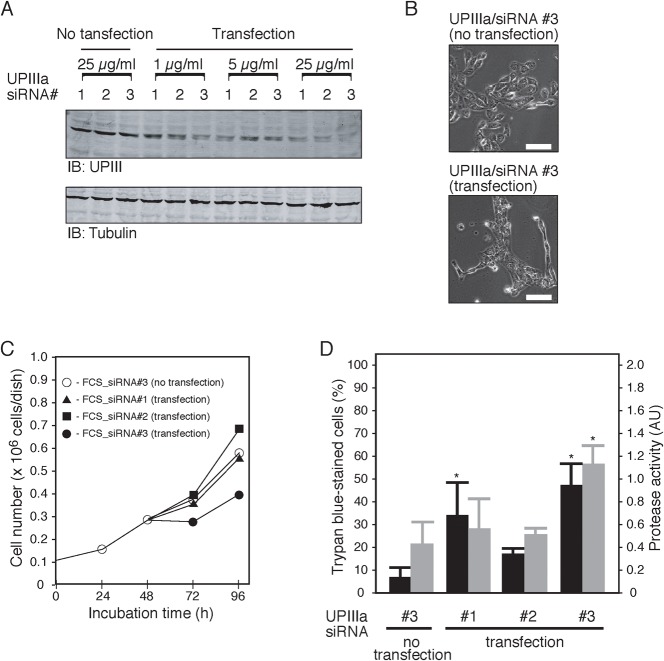
Further support for the importance of UPIIIa was obtained with knockdown experiments. By using small interfering RNA approaches, we prepared 5637 cells transiently down-regulating UPIIIa (Fig. 6A, siRNA no. 3). Those knockdown cells exhibited an altered morphology in their peripheral regions when compared with control cells expressing UPIIIa normally (Fig. 6B), although its molecular insight is unknown. Moreover, knockdown of UPIIIa resulted in a reduced rate of proliferation (Fig. 6C) and enhanced the apoptotic cell death (Fig. 6D, siRNA no. 3). It should also be noted the occurrence depended upon the species of siRNA (Fig. 6B,C, siRNA no. 1 and no. 2).
Fig. 6. Knockdown of UPIIIa attenuates the anti-apoptotic functions of serum-starved 5637 cells.
(A) 5637 cells (2∼4×105 cells per dish) were subjected to transfection treatment with the indicated concentrations (1–25 µg/mL) of three kinds of siRNA for UPIIIa (no. 1–3) for 24 h as described in Materials and Methods. For control experiments, cells were also treated with the same siRNA in the absence of transfection reagents (no transfection). Protein samples (30 µg/lane) of the transfected cells were analyzed by immunoblotting with the anti-UPIII antibody (IB: UPIII) or an anti-tubulin α antibody (IB: tubulin). (B) Morphology of 5637 cells that had been treated with the siRNA for UPIIIa (no. 3, 25 µg/mL) in the absence or the presence of transfection reagents. Scale bars are 50 µm. (C) Cell number of 5637 cells before and after transfection was determined. Transfection treatments, as described above (all 25 µg/mL of siRNAs), were started at 24 h, preceded for 24 h (48 h in the graph), and further cultured in serum-free medium for 48 h (96 h in the graph). (D) Cell death (24 h transfection plus 24 h serum-free culture) and caspase activity (24 h transfection plus 8 h serum-free culture) of the siRNA (25 µg/mL)-treated cells were determined. *P<0.01 compared with control.
Serum starvation promotes up-regulation of heparin-binding EGF-like growth factor and some other genes in an Src-dependent manner
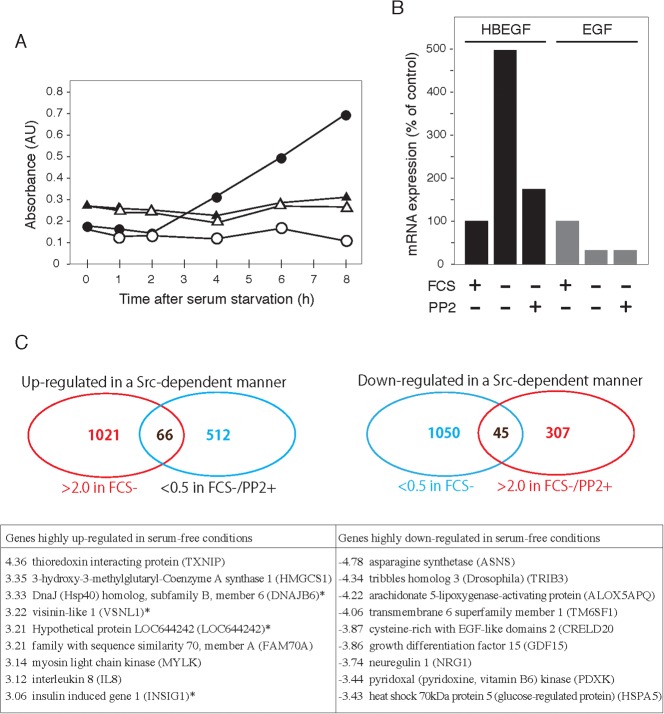
It has been shown that serum-starved 5637 cells promote tyrosine phosphorylation and activation of EGFR (Fig. 3) (Yamamoto et al., 2006) through autocrined secretion of EGF and/or EGF-related ligands (Ruck and Paulie, 1998). Enzyme-lined immunosorbent assays demonstrated that secretion of HB-EGF, but not EGF, was upregulated in the course of serum starvation (Fig. 7A). Analyses of the expression level of mRNA using an Affymetrix Gene-chip array showed that serum starvation of 5637 cells promoted upregulation of HB-EGF in an Src-dependent manner (Fig. 7B). The array analyses also demonstrated a global change of gene expression in serum-starved 5637 cells. 1021 genes were identified to be more than 2-fold up-regulated in serum-starved cells (compared with control cells) (Fig. 7C, list shown on left), and 512 of these 1021 genes were found to be more than 2-fold down-regulated in serum-starved and PP2-containing culture conditions (compared with serum-starved cells). As a result, 66 genes were identified as being up-regulated in an Src-dependent manner (Fig. 7C; supplementary material Fig. S6). On the other hand, 1050 genes were identified as being down-regulated more than 0.5-fold in serum-starved cells (Fig. 7C, list shown on right), and 307 of these 1050 genes were found to be more than 2-fold up-regulated in serum-starved and PP2-containing conditions. As a result, 45 genes were identified as being down-regulated in an Src-dependent manner (Fig. 7C; supplementary material Fig. S7).
Fig. 7. Src-dependent expression of HB-EGF and other genes in serum-starved 5637 cells.
(A) 5637 cells (106 cells per dish) were cultured in serum-free medium in the absence (•, ▴) or the presence (○, ▵) of 5 µM PP2 for the indicated times (0–8 h). At each time point of treatments, culture media were collected, concentrated, and analyzed for the presence of HB-EGF ( , ○) or EGF (▴, ▵) by enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. (B) 5637 cells (106 cells per dish) were cultured in normal (FCS +), serum-free (FCS −), or serum-free plus 5 µM PP2-containing (FCS −, PP2 +) conditions for 8 h. After the treatments, total RNA was prepared and relative expression level of mRNA for HB-EGF (black bars) and EGF (grey bars) was determined by DNA microarray analysis as described in Materials and Methods. (C) Shown in the upper half is the global patterns of Src-dependent gene expression in serum-starved 5637 cells were analyzed by Affimetrix Gene-chip arrays as described in Materials and Methods. According to the criteria described in Results, 66 genes were found to be up-regulated, while 45 genes were found to be down-regulated, both in a Src-dependent manner, in serum-starved 5637 cells. Shown in the bottom half is the top nine list for highly up-regulated or down-regulated genes (irrespective of the dependency on Src activity) (full name and gene ID are shown) in serum-starved 5637 cells. Numbers shown are multiplier factors (n) of 2 (fold change: 2n for up-regulated genes; 2−n for down-regulated genes). Asterisks indicate the genes, whose expression levels reversed back more than 2-fold in serum-free and PP2-contained culture conditions.
, ○) or EGF (▴, ▵) by enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. (B) 5637 cells (106 cells per dish) were cultured in normal (FCS +), serum-free (FCS −), or serum-free plus 5 µM PP2-containing (FCS −, PP2 +) conditions for 8 h. After the treatments, total RNA was prepared and relative expression level of mRNA for HB-EGF (black bars) and EGF (grey bars) was determined by DNA microarray analysis as described in Materials and Methods. (C) Shown in the upper half is the global patterns of Src-dependent gene expression in serum-starved 5637 cells were analyzed by Affimetrix Gene-chip arrays as described in Materials and Methods. According to the criteria described in Results, 66 genes were found to be up-regulated, while 45 genes were found to be down-regulated, both in a Src-dependent manner, in serum-starved 5637 cells. Shown in the bottom half is the top nine list for highly up-regulated or down-regulated genes (irrespective of the dependency on Src activity) (full name and gene ID are shown) in serum-starved 5637 cells. Numbers shown are multiplier factors (n) of 2 (fold change: 2n for up-regulated genes; 2−n for down-regulated genes). Asterisks indicate the genes, whose expression levels reversed back more than 2-fold in serum-free and PP2-contained culture conditions.
HB-EGF plays an important role in signal transduction and anti-apoptosis in serum-starved 5637 cells
An antibody against the HB-EGF had no effect on the proteolysis of UPIIIa; however, it caused inhibition of the activation of Src and promoted apoptotic cell death in serum-starved 5637 cells (Fig. 8A,B). These results suggest that HB-EGF acts as an upstream, positive signal for Src activation in response to serum starvation and that proteolysis of UPIIIa is an independent phenomenon from such a signaling cascade involving HB-EGF (see Discussion). Secretion of HB-EGF was inhibited by GM6001 and consistently, the tyrosine kinase signaling involving phosphorylation of p145met and Src was also impaired under the same conditions (Fig. 8C,D). Inhibition of the secretion of HB-EGF was also observed with the anti-UPIII-ED antibody, PP2, and MβCD treatments (Fig. 8D). These results suggest that proteolysis of UPIIIa, Src activity, and cholesterol-enriched MDs are required for the HB-EGF secretion in response to serum starvation.
Fig. 8. Importance of HB-EGF secretion for survival and growth in serum-starved 5637 cells.
(A,B) 5637 cells were cultured in serum-containing (FCS +) or serum-free (FCS −) medium in the presence of anti-HB-EGF antibody (10 µg/ml IgG) or control antibody (25 µg/ml IgG) as described in Materials and Methods. After the treatments, cell samples were analyzed for (A) the proteolysis of UPIIIa (IB: UPIII) and the activation of Src (IB: pY418-Src), and for (B) cell death and caspase 3/7 activity. (C) 5637 cells were exposed to serum-free medium for 8 h in the presence of either GM6001 (10 µg/ml) or its inactive analog (GM6001 n.c., 10 µg/ml). Triton X-100-solubilized cell extracts were prepared and analyzed for tyrosine phosphorylation of 145met (upper panel) and p60src (lower panel) as in Fig. 1A. Asterisks indicate the positions of phosphorylated forms of p145met (pp145met) and p60src (pp60src). Data obtained with the cell extracts prepared from cells grown in serum-containing medium (FCS +) are also shown. (D) Quantifications of HB-EGF in culture media were done under the indicated conditions as in Fig. 7A.
Discussion
Serum starvation has been widely employed as a means of control treatment of cells whose reactions to a variety of extracellular stimuli (e.g. growth factors) are of interest. During the past several years, however, serum starvation by itself has received much attention from researchers (Pirkmajer and Chibalin, 2011; Tavaluc et al., 2007); namely, it has been employed as a model experimental condition of interest, by which researchers can investigate the behavior of cancer cells under such a stressful environment in vitro. Other experimental conditions that could represent the physiological microenvironment of cancer cells in vivo include hypoxia (low O2 concentration), lack of cell adhesion to substratum (leading to anoikis in normal cells), and low nutrient (e.g. depletion of glucose). Under these stressful conditions, cancer cells with high malignancy somehow acquire the ability to evade the onset of apoptosis. Our previous study using the bladder carcinoma cell 5637 demonstrated that the tyrosine kinase signaling pathway involving Src, EGFR, and Met acts as an anti-apoptotic mechanism that allows the cells to proliferate under serum-starved culture conditions (Yamamoto et al., 2006). On the other hand, other types of human cancer cells employ Akt protein kinase (Huang et al., 2011; for a review, see Hemmings, 1997), protein kinase Cδ (Chen et al., 2010), glycogen synthase kinase 3β (Yang et al., 2010), AAA-ATPase (Fang et al., 2010), and survivin (Islam et al., 2000) as an anti-apoptotic or anti-death mechanism under serum-starved conditions. This background led us to examine further the role of tyrosine kinase signaling pathway in 5637 and other bladder carcinoma cells.
In this study, we show that serum-independent proliferation operates not only in 5637 cells but also in some other bladder carcinoma cells (HT1197 and HT1376) (Fig. 1). In these cells, serum starvation promotes Src activation and no MAPK activation, and consistently, PP2, a pharmacological Src inhibitor, but not U0126, an inhibitor of MAPK kinase (MEK), blocks the cell proliferation and lead to apoptosis under serum-starved conditions. It should be noted that elevation of activating phosphorylation on tyrosine-418 of Src is always observed in serum-independent proliferation-positive cells, whereas a decrease in inhibitory phosphorylation on tyrosine-529 is hardly detectable in these cells, suggesting a phosphatase-independent, but UPIIIa-dependent, mechanism of Src activation (see below). Forced expression of kinase-inactive Src mutant also abolishes the proliferation and promotes apoptosis of serum-starved 5637 cells (Fig. 2). These results suggest that anti-apoptotic serum-independent proliferation of bladder carcinoma cells involves Src-dependent and MAPK-independent mechanisms, whereas the opposite mechanisms (i.e. Src-independent and MAPK-dependent) seem to work under serum-containing conditions (supplementary material Fig. S2). On the other hand, we did not detect 5637 cell-like functions in other types of human cancer or immortalized cells (A431, ACHN, HEK293, HT29, and MCF7) (Fig. 1; supplementary material Fig. S1). Thus, the Src-dependent and MAPK-independent mechanisms of anti-apoptosis under serum-starved conditions seem to be a specific feature of bladder carcinoma cells.
As a structural platform for Src tyrosine kinase signaling in 5637 cells, we have identified and characterized cholesterol-enriched MDs, where Src, EGFR, and Met are stably or transiently localized and activated in response to serum starvation (Fig. 3). Serum-starved 5637 cells undergo apoptosis in the presence of MβCD, a drug that disrupts the cholesterol-dependent structure and function of MDs. MβCD is also shown to abolish the MD localization of Src, EGFR, and Met and their activating phosphorylations (Fig. 3). It should be noted that the protein expression level of these signaling molecules is not altered in MβCD-treated cells, suggesting that their MD localizations are important for signal transduction and cell behavior. Several lines of evidence indicate that, in a variety of cells and tissues, and irrespective of physiological and pathological situations, MDs and their associated molecules (e.g. proteins, lipids) play an important role in sensing extracellular environmental signals and triggering the cellular responses (Brown and London, 1998; Simons and Toomre, 2000). Although it is currently unknown how serum starvation is sensed and translated to signal transduction in 5637 cells (see below), our present study demonstrates for the first time the importance of MDs in this kind of signal transduction.
Cellular functions of MDs often require a certain kind of membrane protein(s) (e.g. caveolin, tetraspanin, and their associated proteins) that acts as a membrane organizer (Anderson, 1998; Hemler, 2005). In this study, we have identified UPIIIa, an urothelium-specific, type I transmembrane protein that is a member of the UP protein family, as an MD-associated component of serum starvation-induced Src tyrosine kinase signaling leading to anti-apoptotic proliferation in 5637 cells. Like Src and Met, UPIIIa stably localizes to MDs irrespective of serum conditions in a cholesterol-dependent manner (Fig. 4). Further experiments demonstrated that UPIIIa undergoes partial proteolysis in response to serum starvation (Fig. 4), and that the inhibition of the proteolysis by a specific antibody to the extracellular domain of UPIIIa or an MMP inhibitor GM6001 results in failure of Src activation and the occurrence of apoptosis (Fig. 5). Moreover, knockdown of UPIIIa by short interfering RNA also promotes apoptosis of serum-starved 5637 cells (Fig. 6). From these data, we conclude that UPIIIa is involved in Src-dependent signal transduction for survival and proliferation of 5637 cells under serum-starved conditions.
UPIIIa was originally identified in mammals, including humans, as a component of AUMs that are made of clusters of 16-nm crystal structures in the apical surface of water-impermeable urothelial cell membranes (Jenkins and Woolf, 2007; Khandelwal et al., 2009; Liang et al., 2001; Wu et al., 2009; Wu and Sun, 1993). In the structures of AUMs, all four members of the UP family protein are indispensable: UPIIIa forms a heterodimer with uroplakin Ib (UPIb), a tetraspanin molecule, and the UPIb-UPIIIa complex associates further with UPIa (tetraspanin)-UPII (type I transmembrane protein) complex (Liang et al., 2001; Wu et al., 1994; Wu et al., 1990). On the other hand, the role of individual UP family proteins in bladder cell function has also been demonstrated recently (Lobban et al., 1998; Moll et al., 1995). In particular, the following cellular features and/or functions of mammalian UPIIIa other than for urothelial membrane architecture (Hu et al., 2000) have been reported: 1) it can be used as a urinary marker for the detection of bladder cancer (Lai et al., 2010), 2) its expression level inversely correlates with the malignancy grade of bladder cancers (Fig. 4A) (Koga et al., 2003; Matsumoto et al., 2008; Ohtsuka et al., 2006; Parker et al., 2003), 3) mutation in the UPIIIa sequence is often associated with the occurrence of some renal diseases (Jenkins et al., 2005; Schönfelder et al., 2006), and 4) it is involved in urothelial cell death or casein kinase II-dependent phospho-signal transduction in response to uropathogenic bacterial infection (Thumbikat et al., 2009a; Thumbikat et al., 2009b). In addition, studies using the African clawed frog Xenopus laevis have demonstrated that fertilization triggers partial proteolysis and tyrosine phosphorylation of UPIIIa in egg, and that the egg UPIIIa associates with UPIb and localizes to MDs, and thus may be involved in sperm-induced activation of Src tyrosine kinase signaling (Mahbub Hasan et al., 2011; Mahbub Hasan et al., 2007; Mahbub Hasan et al., 2005; Sakakibara et al., 2005). Taken these findings into account, it is not so surprising that UPIIIa, through its MD localization and partial proteolysis (but not tyrosine phosphorylation), is involved in the Src-dependent signal transduction for anti-apoptosis in serum-starved 5637 cells.
Currently, it is not known how functions of UPIIIa (partial proteolysis) and Src in 5637 cells are connected. In the case of Xenopus egg system, it has been suggested that, in unfertilized eggs, the UPIb/UPIIIa complex negatively regulates the activity of Src and that, upon fertilization, negative regulation of Src is canceled by the action of sperm-derived tryptic protease (Mahbub Hasan et al., 2011; Mahbub Hasan et al., 2007; Mahbub Hasan et al., 2005). In this case, proteolysis of UPIIIa does not require Src activity (signal flows mono-directionally from UPIIIa-like protein to Src). In serum-starved 5637 cells, application of an antibody against the extracellular domain of UPIIIa blocks the proteolysis of UPIIIa and activation of Src, suggesting that the proteolysis of UPIIIa occurs in a similar manner to that seen in Xenopus egg. However, neither inhibitors nor a synthetic substrate for tryptic protease block the proteolysis of UPIIIa (supplementary material Fig. S4). Instead, GM6001, a broad-specificity inhibitor of MMP, shows a strong inhibitory effect on UPIIIa proteolysis, Src activation, and cell survival and proliferation (Fig. 5). Thus, partial proteolysis of UPIIIa in serum-starved 5637 cells seems to involve MMP activity. Interestingly, Src inhibitor PP2 also blocks proteolysis of UPIIIa (supplementary material Fig. S5), suggesting that proteolysis of UPIIIa and activation of Src constitute a positive feedback loop (signal flows bi-directionally between UPIIIa and Src) (Fig. 9).
Fig. 9. A schematic drawing of the Src-dependent anti-apoptotic mechanisms of serum-independent proliferation in 5637 bladder carcinoma cells.
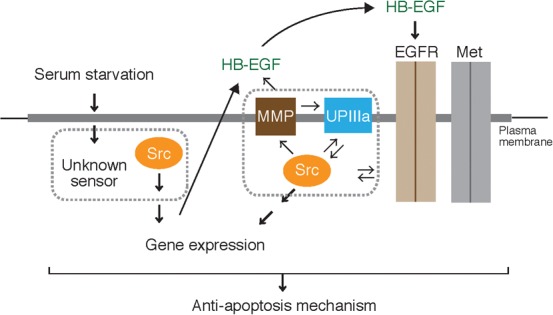
Cells recognize or sense the signal of serum-starved conditions by unknown mechanism and then promote the activation of Src. Gene expression and secretion of HB-EGF is stimulated in a Src and MMP-dependent manner. HB-EGF, as a ligand, activates EGFR. The activated EGFR, in corporation with Src, contributes to the tyrosine phosphorylation of p145met. UPIIIa, a newly characterized protein in this study, undergoes partial proteolysis in an MMP (and maybe Src)-dependent manner under serum-starved conditions, by which it contributes to the activation of Src. Cholesterol-enriched MDs, as indicated by the grey area on the plasma membranes, serve as a platform for the signaling network as described above. For detail, see Discussion.
It is well known that extracellular secretion of EGF ligands such as EGF and HB-EGF depends on the activity of MMPs that process the immature and membrane-anchored forms of EGF and HB-EGF (Higashiyama et al., 2008; Miyamoto et al., 2006; Sanderson et al., 2006). In fact, we demonstrated that expression and secretion of HB-EGF, but not EGF, is up-regulated in serum-starved 5637 cells in a GM6001-sensitive manner (Figs 7, 8). Further characterization of HB-EGF employing its neutralizing antibody as well as aforementioned experimental conditions demonstrates that HB-EGF is an important factor for the Src-dependent anti-apoptosis mechanism (Fig. 8). Moreover, it is suggested that MMP-dependent production of HB-EGF and Src activation constitute a positive feedback loop (Fig. 9). On the other hand, blockage of HB-EGF does not result in inhibition of partial proteolysis of UPIIIa, whereas specific inhibition of UPIIIa proteolysis attenuates the secretion of HB-EGF (maybe because of Src inhibition). Taking these findings in together, we suggest that the anti-apoptotic mechanism of 5637 cells under serum-starved conditions involves a signal transduction system of hitherto unknown combination, that is, activity of MMPs, HB-EGF, UPIIIa, and Src, all of which are connected with each other (Fig. 9). DNA microarray analysis carried out in this study demonstrates that expression of a number of genes may be up- or down-regulated under the control of Src activity (Fig. 7; supplementary material Figs S6, S7). In future study, we will examine how the membrane-associated, non-genomic signaling machinery, mainly highlighted in the present study, interacts with the nuclear, genomic regulatory system. This kind of study will also involve the characterization of sensing system for serum starvation, possibly metabolic regulatory machinery in anti-apoptosis of cancer cells.
Supplementary Material
Acknowledgments
We thank the former and current members of our laboratories. This work is supported in part by a Grant-in-Aid for Scientific Research (B), Japan Society for the Promotion of Science (19370094), a grant for Private University Strategic Research Foundation Support Program (S0801060), and a Grant-in-Aid on Innovative Areas, Ministry of Education, Culture, Sports, Science, and Technology, Japan (22112522) to K.S.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Aleshin A., Finn R. S. (2010). SRC: a century of science brought to the clinic. Neoplasia 12, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G. (1998). The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225 10.1146/annurev.biochem.67.1.199 [DOI] [PubMed] [Google Scholar]
- Brown D. A., London E. (1998). Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136 10.1146/annurev.cellbio.14.1.111 [DOI] [PubMed] [Google Scholar]
- Chen C. L., Chan P. C., Wang S. H., Pan Y. R., Chen H. C. (2010). Elevated expression of protein kinase Cδ induces cell scattering upon serum deprivation. J. Cell Sci. 123, 2901–2913 10.1242/jcs.069765 [DOI] [PubMed] [Google Scholar]
- Fang H. Y., Chang C. L., Hsu S. H., Huang C. Y., Chiang S. F., Chiou S. H., Huang C. H., Hsiao Y. T., Lin T. Y., Chiang I. P.et al. (2010). ATPase family AAA domain-containing 3A is a novel anti-apoptotic factor in lung adenocarcinoma cells. J. Cell Sci. 123, 1171–1180 10.1242/jcs.062034 [DOI] [PubMed] [Google Scholar]
- Griffin J. L., Shockcor J. P. (2004). Metabolic profiles of cancer cells. Nat. Rev. Cancer 4, 551–561 10.1038/nrc1390 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- Harris A. L. (2002). Hypoxia-a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- Hemler M. E. (2005). Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 10.1038/nrm1736 [DOI] [PubMed] [Google Scholar]
- Hemmings B. A. (1997). Akt signaling: linking membrane events to life and death decisions. Science 275, 628–630 10.1126/science.275.5300.628 [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Iwabuki H., Morimoto C., Hieda M., Inoue H., Matsushita N. (2008). Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 99, 214–220 10.1111/j.1349-7006.2007.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Deng F. M., Liang F. X., Hu C. M., Auerbach A. B., Shapiro E., Wu X. R., Kachar B., Sun T. T. (2000). Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J. Cell Biol. 151, 961–972 10.1083/jcb.151.5.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B. X., Akbar M., Kevala K., Kim H. Y. (2011). Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 192, 979–992 10.1083/jcb.201005100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (2009). Tyrosine phosphorylation: thirty years and counting. Curr. Opin. Cell Biol. 21, 140–146 10.1016/j.ceb.2009.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Lane H. A. (2005). ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 10.1038/nrc1609 [DOI] [PubMed] [Google Scholar]
- Islam A., Kageyama H., Takada N., Kawamoto T., Takayasu H., Isogai E., Ohira M., Hashizume K., Kobayashi H., Kaneko Y.et al. (2000). High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene 19, 617–623 10.1038/sj.onc.1203358 [DOI] [PubMed] [Google Scholar]
- Jenkins D., Woolf A. S. (2007). Uroplakins: new molecular players in the biology of urinary tract malformations. Kidney Int. 71, 195–200 10.1038/sj.ki.5002053 [DOI] [PubMed] [Google Scholar]
- Jenkins D., Bitner-Glindzicz M., Malcolm S., Hu C. C., Allison J., Winyard P. J., Gullett A. M., Thomas D. F., Belk R. A., Feather S. A.et al. (2005). De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J. Am. Soc. Nephrol. 16, 2141–2149 10.1681/ASN.2004090776 [DOI] [PubMed] [Google Scholar]
- Khandelwal P., Abraham S. N., Apodaca G. (2009). Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol. 297, F1477–F1501 10.1152/ajprenal.00327.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga F., Kawakami S., Fujii Y., Saito K., Ohtsuka Y., Iwai A., Ando N., Takizawa T., Kageyama Y., Kihara K. (2003). Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin. Cancer Res. 9, 5501–5507. [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lai Y., Ye J., Chen J., Zhang L., Wasi L., He Z., Zhou L., Li H., Yan Q., Gui Y.et al. (2010). UPK3A: a promising novel urinary marker for the detection of bladder cancer. Urology 76, 514.e6–514.e11 10.1016/j.urology.2009.11.045 [DOI] [PubMed] [Google Scholar]
- Liang F. X., Riedel I., Deng F. M., Zhou G., Xu C., Wu X. R., Kong X. P., Moll R., Sun T. T. (2001). Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem. J. 355, 13–18 10.1042/0264-6021:3550013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobban E. D., Smith B. A., Hall G. D., Harnden P., Roberts P., Selby P. J., Trejdosiewicz L. K., Southgate J. (1998). Uroplakin gene expression by normal and neoplastic human urothelium. Am. J. Pathol. 153, 1957–1967 10.1016/S0002-9440(10065709-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbub Hasan A. K. M., Sato K., Sakakibara K., Ou Z., Iwasaki T., Ueda Y., Fukami Y. (2005). Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization. Dev. Biol. 286, 483–492 10.1016/j.ydbio.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Mahbub Hasan A. K. M., Ou Z., Sakakibara K., Hirahara S., Iwasaki T., Sato K., Fukami Y. (2007). Characterization of Xenopus egg membrane microdomains containing uroplakin Ib/III complex: roles of their molecular interactions for subcellular localization and signal transduction. Genes Cells 12, 251–267 10.1111/j.1365-2443.2007.01048.x [DOI] [PubMed] [Google Scholar]
- Mahbub Hasan A. K. M., Fukami Y., Sato K. (2011). Gamete membrane microdomains and their associated molecules in fertilization signaling. Mol. Reprod. Dev. 78, 814–830 10.1002/mrd.21336 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Satoh T., Irie A., Ishii J., Kuwao S., Iwamura M., Baba S. (2008). Loss expression of uroplakin III is associated with clinicopathologic features of aggressive bladder cancer. Urology 72, 444–449 10.1016/j.urology.2007.11.128 [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Yagi H., Yotsumoto F., Kawarabayashi T., Mekada E. (2006). Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci. 97, 341–347 10.1111/j.1349-7006.2006.00188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Wu X. R., Lin J. H., Sun T. T. (1995). Uroplakins, specific membrane proteins of urothelial umbrella cells, as histological markers of metastatic transitional cell carcinomas. Am. J. Pathol. 147, 1383–1397. [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Kawakami S., Fujii Y., Koga F., Saito K., Ando N., Takizawa T., Kageyama Y., Kihara K. (2006). Loss of uroplakin III expression is associated with a poor prognosis in patients with urothelial carcinoma of the upper urinary tract. BJU Int. 97, 1322–1326 10.1111/j.1464-410X.2006.06158.x [DOI] [PubMed] [Google Scholar]
- Parker D. C., Folpe A. L., Bell J., Oliva E., Young R. H., Cohen C., Amin M. B. (2003). Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am. J. Surg. Pathol. 27, 1–10 10.1097/00000478-200301000-00001 [DOI] [PubMed] [Google Scholar]
- Pirkmajer S., Chibalin A. V. (2011). Serum starvation: caveat emptor. Am. J. Physiol. Cell Physiol. 301, C272–C279 10.1152/ajpcell.00091.2011 [DOI] [PubMed] [Google Scholar]
- Ruck A., Paulie S. (1997). The epidermal growth factor receptor is involved in autocrine growth of human bladder carcinoma cell lines. Anticancer Res. 17, 1925–1931. [PubMed] [Google Scholar]
- Ruck A., Paulie S. (1998). EGF, TGF alpha, AR and HB-EGF are autocrine growth factors for human bladder carcinoma cell lines. Anticancer Res. 18, 1447–1452. [PubMed] [Google Scholar]
- Ruck A., Jakobson E., Björkman S., Paulie S. (1994). Adaptation of human bladder carcinoma cell lines to serum-free growth. Evidence for autocrine growth stimulation. Anticancer Res. 14, 55–60. [PubMed] [Google Scholar]
- Sakakibara K., Sato K., Yoshino K., Oshiro N., Hirahara S., Mahbub Hasan A. K. M., Iwasaki T., Ueda Y., Iwao Y., Yonezawa K.et al. (2005). Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J. Biol. Chem. 280, 15029–15037 10.1074/jbc.M410538200 [DOI] [PubMed] [Google Scholar]
- Sanderson M. P., Dempsey P. J., Dunbar A. J. (2006). Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24, 121–136 10.1080/08977190600634373 [DOI] [PubMed] [Google Scholar]
- Sato K., Sato A., Aoto M., Fukami Y. (1995). c-Src phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem. Biophys. Res. Commun. 215, 1078–1087 10.1006/bbrc.1995.2574 [DOI] [PubMed] [Google Scholar]
- Schönfelder E. M., Knüppel T., Tasic V., Miljkovic P., Konrad M., Wühl E., Antignac C., Bakkaloglu A., Schaefer F., Weber S.et al. (2006). Mutations in Uroplakin IIIA are a rare cause of renal hypodysplasia in humans. Am. J. Kidney Dis. 47, 1004–1012 10.1053/j.ajkd.2006.02.177 [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 10.1038/35036052 [DOI] [PubMed] [Google Scholar]
- Tavaluc R. T., Hart L. S., Dicker D. T., El-Deiry W. S. (2007). Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle 6, 2554–2562 10.4161/cc.6.20.4911 [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. (1997). Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 10.1146/annurev.cellbio.13.1.513 [DOI] [PubMed] [Google Scholar]
- Thumbikat P., Berry R. E., Schaeffer A. J., Klumpp D. J. (2009a). Differentiation-induced uroplakin III expression promotes urothelial cell death in response to uropathogenic E. coli. Microbes Infect. 11, 57–65 10.1016/j.micinf.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumbikat P., Berry R. E., Zhou G., Billips B. K., Yaggie R. E., Zaichuk T., Sun T. T., Schaeffer A. J., Klumpp D. J. (2009b). Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 5, e1000415 10.1371/journal.ppat.1000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. R., Sun T. T. (1993). Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J. Cell Sci. 106, 31–43. [DOI] [PubMed] [Google Scholar]
- Wu X. R., Manabe M., Yu J., Sun T. T. (1990). Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J. Biol. Chem. 265, 19170–19179. [PubMed] [Google Scholar]
- Wu X. R., Lin J. H., Walz T., Häner M., Yu J., Aebi U., Sun T. T. (1994). Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J. Biol. Chem. 269, 13716–13724. [PubMed] [Google Scholar]
- Wu X. R., Kong X. P., Pellicer A., Kreibich G., Sun T. T. (2009). Uroplakins in urothelial biology, function, and disease. Kidney Int. 75, 1153–1165 10.1038/ki.2009.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Mammadova G., Song R. X., Fukami Y., Sato K. (2006). Tyrosine phosphorylation of p145met mediated by EGFR and Src is required for serum-independent survival of human bladder carcinoma cells. J. Cell Sci. 119, 4623–4633 10.1242/jcs.03236 [DOI] [PubMed] [Google Scholar]
- Yang J., Takahashi Y., Cheng E., Liu J., Terranova P. F., Zhao B., Thrasher J. B., Wang H. G., Li B. (2010). GSK-3beta promotes cell survival by modulating Bif-1-dependent autophagy and cell death. J. Cell Sci. 123, 861–870 10.1242/jcs.060475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.