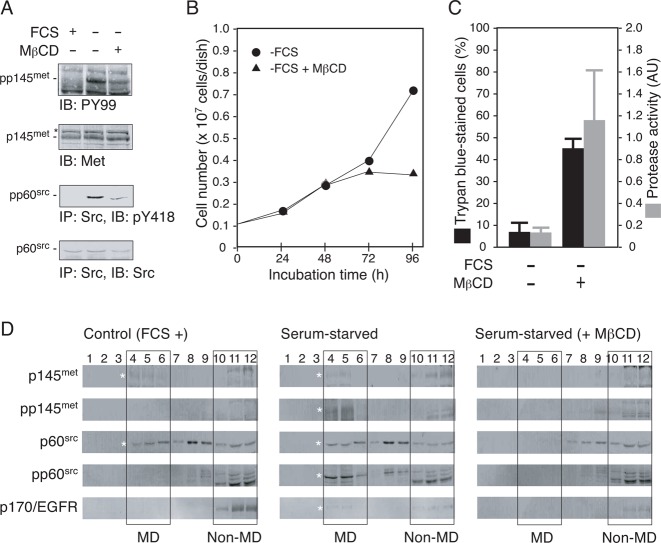
Fig. 3. Disruption of cholesterol-dependent MDs interferes with Src-dependent signal transduction and promotes apoptosis in serum-starved 5637 cells.
(A) 5637 cells were grown in serum-free condition in the absence or the presence of methyl-β-cyclodextrin (MβCD, 15 µM) for 8 h. After the treatments, Triton X-100-solubilized cell extracts were prepared and analyzed for tyrosine phosphorylation of p145met and p60src by immunoprecipitation (300 µg/lane) and/or immunoblotting (30 µg/lane). Control data obtained with the extracts prepared from cells grown in serum-containing medium (FCS +) are also shown. (B) 5637 cells (0.1×107 cells/dish) were grown in serum-containing condition for 48 h and then exposed to serum-free medium with (▴) or without ( ) 15 µM MβCD for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 2B. (C) 5637 cells were cultured in serum-containing (FCS +) or serum-free (FCS −) medium with or without 15 µM MβCD as in panel B for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. (D) 5637 cells were cultured in serum-containing medium (control, FCS +), serum-free medium in the absence (serum-starved) or the presence of 15 µM MβCD (serum-starved, + MβCD). Cell samples were subjected to subcellular fractionation by discontinuous sucrose density gradient ultracentrifugation as described in Materials and Methods. Twelve fractions obtained were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Rectangles indicate the positions of low density, detergent-insoluble MD fractions (fractions 4–6, denoted as “MD”) and non-MD fractions (fractions 10–12, denoted as “non-MD”). Asterisks indicate the positions of proteins of interest.
) 15 µM MβCD for 48 h (total incubation time of 96 h). At 24, 48, 72, and 96 h of treatments, cell number was determined as in Fig. 2B. (C) 5637 cells were cultured in serum-containing (FCS +) or serum-free (FCS −) medium with or without 15 µM MβCD as in panel B for 8 h. After the treatments, cell death and caspase 3/7 activity were determined as in Fig. 2C. (D) 5637 cells were cultured in serum-containing medium (control, FCS +), serum-free medium in the absence (serum-starved) or the presence of 15 µM MβCD (serum-starved, + MβCD). Cell samples were subjected to subcellular fractionation by discontinuous sucrose density gradient ultracentrifugation as described in Materials and Methods. Twelve fractions obtained were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Rectangles indicate the positions of low density, detergent-insoluble MD fractions (fractions 4–6, denoted as “MD”) and non-MD fractions (fractions 10–12, denoted as “non-MD”). Asterisks indicate the positions of proteins of interest.