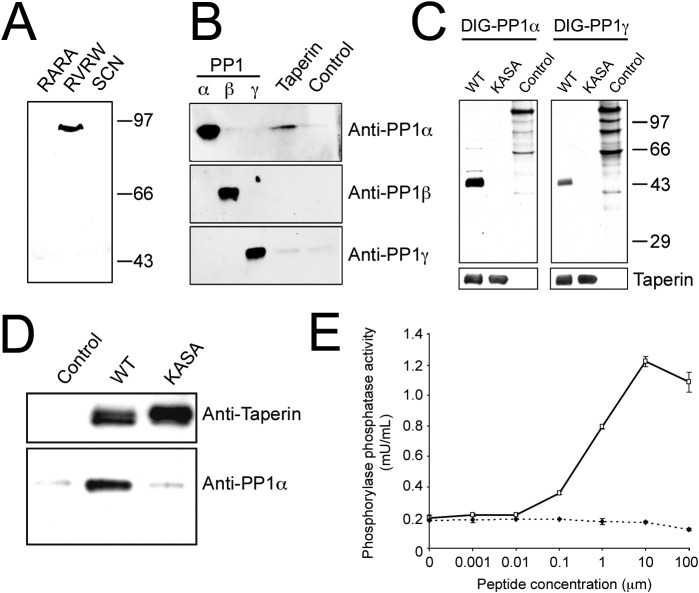
Fig. 1. Taperin (c9orf75) preferentially associates with PP1α.
(A) HeLa cell nuclear extract was incubated with microcystin-Sepharose matrix, washed extensively and sequentially eluted with first the RARA peptide, followed by the RVRW peptide to specifically elute PP1 binding complexes, and finally a 3 M NaSCN step to elute any remaining protein. Eluates were then subjected to SDS-PAGE, transferred to nitrocellulose and a Western blot performed using affinity purified taperin antibodies (2 µg/mL). Endogenous taperin was immunoprecipitated (B) from a HeLa cell extract and blotted with the PP1 isoform specific antibody shown on the right. Control immunoprecipitation was with an equivalent amount of pre-immune IgG. Antibody specificity is shown by running 10 ng, 10 ng and 2 ng of recombinant purified PP1α, β and γ respectively, and as shown in supplementary material Fig. S12 and S13. (C) for far-Western blots, proteins were subjected to SDS-PAGE, transferred to nitrocellulose and overlaid with recombinant digoxigenin-coupled PP1α or PP1γ. Proteins are wild type [KISF] or mutated [KASA] 6His-taperin and 30 µg of rat nuclear extract as control. To ensure equal loading membranes were stripped and re-probed with anti-taperin (lower panels). For pulldowns (D), recombinant PP1α was mixed with Ni-NTA beads alone (control) or with recombinant wild type (KISF) or mutated (KASA) 6His-taperin. After release from the beads with SDS sample buffer, proteins were subjected to Western blot analysis with anti-taperin or anti-PP1 antibodies, as indicated. (E) the protein phosphatase activity of PP1α was monitored when mixed with an equal molar amount of taperin in the presence of increasing amounts of either RVRW peptide (—□—; GKKRVRWADLE) or RARA peptide (—♦—; GKKRARAADLE). Protein phosphatase activity was measured using phosphorylase a as the substrate. Data points are mean ± SD for n = 3.