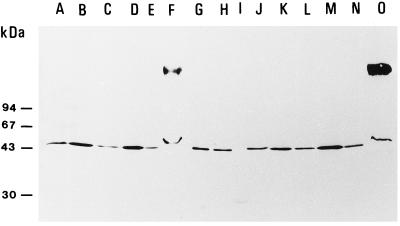
Figure 4.
Immunoblotting of the PM and supernatant fractions of the MDA reductase activity fractionation experiment using anti-MDA reductase antibodies. Lanes A to F, Fractions from PM preparation with Suc in the homogenization buffer; lanes J to O, fractions from PM preparation with KCl in the homogenization buffer. A and J, PM; B and K, S1 fraction; C and L, S2 fraction; D and M, S3 fraction; E and N, S4 fraction; F and O, S5 fraction; G and H, Mono-Q fraction Q27 and affinity fraction A40 (Bérczi et al., 1995); and I, standard proteins. About the same total MDA reductase activity (approximately 5 nmol min−1) was applied in each lane (except lane I). The positions of standard proteins of known molecular mass are shown on the left. All fractions were from the same experiment. Three independent fractionation experiments gave similar results. The high-molecular-mass band in lanes F and O is an artifact probably caused by CHAPS.