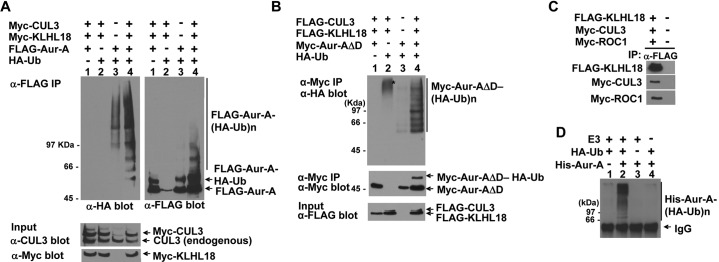
Fig. 8. The CUL3-KLHL18 ligase ubiquitylates Aurora-A in vivo and in vitro.
(A, B) FLAG-tagged Aurora-A (A) or Myc-tagged Aurora-A deleted C-terminal D-box (Aur-AΔD) (B) were cotransfected with Myc-tagged or FLAG-tagged CUL3, Myc-tagged or FLAG-tagged KLHL18, and HA-tagged Ubiquitin expressing plasmids to 293T cells as indicated. Twenty four hours after transfection, cells were lysed with a 1% SDS containing lysis buffer and boiled for 15 min. Denatured lysates were then diluted with 0.5% NP-40 lysis buffer and IP with anti-FLAG (A) or anti-Myc (B) antibody. The washed immunoprecipitates and the total cell lysate (input) were resolved by SDS-PAGE, followed by WB with indicated antibodies. (C) CUL3-KLHL18-ROC1 complexes were prepared from triply transfected 293T cells by IP using anti-FLAG antibody and used as the source of E3 ligase. (D) Bacterially expressed and purified His-tagged Aurora-A (His-Aur-A) (Fig. 6E) was incubated with the KLHL18-CUL3-ROC1 E3 complex (C), E1, E2, HA-tagged ubiquitin (HA-Ub), and ATP. The reactions were immunoprecipitated with anti-HA antibody and blotted with anti-Aurora-A antibody to examine Aurora-A ubiquitylation.