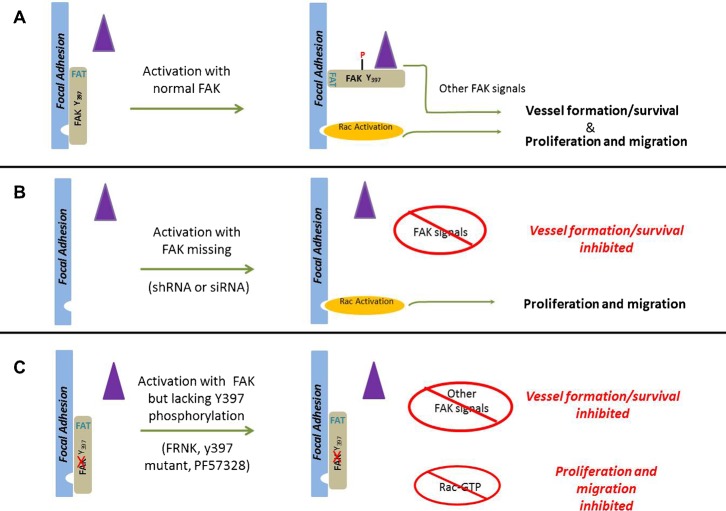
Fig. 9. A model of FAK as a phospho-regulated repressor of Rac activation and endothelial cell function.
(A) Under resting conditions FAK is bound to focal adhesion proteins through FAT-dependent interactions but remain unphosphorylated. Under these conditions Rac activation (orange ellipse) is not efficiently coupled, neither are other signals requiring SRC docking, subsequent phosphorylation and the scaffolding of other signaling proteins (e.g., CAS, Grb7, PI3′-Kinase, Grb2 etc.) (purple triangle). Activation of FAK, results in phosphorylation of tyrosine 397, with subsequent changes in conformation of FAK itself and/or binding partners that permits Rac activation, proliferation and migration. Other signals essential for vascular morphogenesis and survival are also coordinately activated. (B) When FAK is silenced, the physical loss of FAK removes the repression allowing full activation of Rac (upon appropriate stimulation) as well as normal proliferation and migration, even in the absence of FAK397 phosphorylation. However, coordination and activation of the additional signals required for vascular morphogenesis, which are FAK-dependent, is impaired. (C) When FAK is present but is inhibited (whether induced by chemical inhibitors or expression of mutant constructs), the FAT domain of FAK targets FAK but tyrosine 397 phosphorylation is disrupted. Under these conditions the repressive signal cannot be relieved and there is no efficient Rac activation. The altered 397 phosphorylation also disrupts the activation of the FAK-dependent additional signals required for vascular morphogenesis and survival. The loss of focal adhesion targeting via the FAT domain renders these mutants ineffective. Expression of these mutants in cells knocked down for FAK results in repression of Rac activation, proliferation and migration.