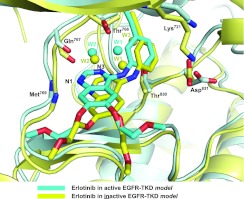
Figure 1. Comparison of erlotinib binding to active and inactive EGFR-TKD models.
On the basis of the computational models described in the present study, erlotinib is shown bound to active EGFR-TKD (cyan) and inactive EGFR-TKD (yellow). The protein structure in the background is similarly coloured (cyan for active, yellow for inactive). Functional groups in several EGFR-TKD residues that interact directly or indirectly with the bound erlotinib are shown. The backbone amide of Met769 donates a hydrogen bond to N1 of the erlotinib quinazoline moiety. The backbone carbonyl of Gln767 and side chains of Thr766 and Thr830 participate in a hydrogen-bonding network to which water molecules (W1 and W2) also contribute. The Lys721 and Asp831 side chains are shown for reference. Polypeptide in the foreground has been removed for clarity.