Abstract
Cells sense physical properties of their extracellular environment and translate them into biochemical signals. In this study, cell responses to surfaces with submicron topographies were investigated in cultured human NF1 haploinsufficient fibroblasts. Age-matched fibroblasts from 8 patients with neurofibromatosis type 1 (NF1+/−) and 9 controls (NF1+/+) were cultured on surfaces with grooves of 200 nm height and lateral distance of 2 μm. As cellular response indicator, the mean cell orientation along microstructured grooves was systematically examined. The tested NF1 haploinsufficient fibroblasts were significantly less affected by the topography than those from healthy donors. Incubation of the NF1+/− fibroblasts with the farnesyltransferase inhibitor FTI-277 and other inhibitors of the neurofibromin pathway ameliorates significantly the cell orientation. These data indicate that NF1 haploinsufficiency results in an altered response to specific surface topography in fibroblasts. We suggest a new function of neurofibromin in the sensoric mechanism to topographies and a partial mechanosensoric blindness by NF1 haploinsufficiency.
Key Words: Mechanosensoric, Neurofibromatosis type 1, Neurofibromin, Topography
Neurofibromatosis type 1 (NF1) is a common monogenic tumor-predisposition disorder caused by inactivating germ line mutations in the neurofibromin gene (NF1+/−) [Ferner, 2007]. Multiple benign nerve sheath tumors, called cutaneous neurofibromas, are one of the hallmarks of the disease. The development of a cutaneous neurofibroma is caused by the bi-allelic inactivation of NF1 (NF1−/−) in a specific progenitor cell which is subsequently clonally expanded [Jouhilahti et al., 2011]. The bi-allelic inactivation of NF1 (NF1-diploinsufficiency) is due to a stochastic inactivating (second) mutation in the NF1 wild-type allele pointing to the tumor suppressor function of NF1 [Parrinello and Lloyd, 2009; Gottfried et al., 2010]. In human diseases related to tumor-suppressor genes, it is often suggested that only the complete loss of the protein caused by the bi-allelic inactivation of a tumor-suppressor gene results in specific symptoms such as tumor formation, whereas simple reduction of protein quantity to about 50% caused by the inactivating germ line mutation, often called haploinsufficiency, essentially does not affect cellular behavior.
Several functional consequences of NF1 haploinsufficiency were characterized in the last decades [McLaughlin and Jacks, 2002; Jouhilahti et al., 2011]. In vivo, NF1 haploinsufficiency is related to several non-tumor manifestations, such as learning and spatial-visual deficits [Hyman et al., 2005], generalized hyperpigmentation [Riccardi, 1981], reduced bone density [Kuorilehto et al., 2005; Lammert et al., 2005], reduced density of dendritic spines [Lin et al., 2007], and the excessive vessel wall cell proliferation after vascular injury [Xu et al., 2007; Lasater et al., 2008]. In addition, NF1 haploinsufficiency in the cellular environment of a tumor promotes its progression in vivo. That observation was made in Nf1 mouse models in both plexiform neurofibromas [Yang et al., 2008; Staser et al., 2012] and optic gliomas [Daginakatte and Gutmann, 2007; Simmons et al., 2011], e.g. via hyperactivated Nf1+/− mast cells as critical mediators [Khalaf et al., 2007; Parrinello and Lloyd, 2009; Staser et al., 2010]. In neurofibromas, a multipotent NF1+/− precursor cell population seems to be important for tumor development [Jouhilahti et al., 2011]. In vitro, NF1 haploinsufficiency influences several cellular pathways. An altered proliferation was found in mast cells [Ingram et al., 2000], melanocytes [Kaufmann et al., 1991], fibroblasts, keratinocytes, osteoblasts, astrocytes [Jouhilahti et al., 2011], and endothelial cells [Wu et al., 2006]. The upregulated cell cycle and DNA repair pathways in NF1+/− cells may promote the loss of the wild-type allele [Pemov et al., 2010]. NF1 haploinsufficiency also downregulates cell susceptibility to apoptosis [Shapira et al., 2007]. In mast cells, it increases the survival in response to Steel factor [Ingram et al., 2000; McDaniel et al., 2008]. Cell-type specific syntheses can be changed by NF1 haploinsufficiency, e.g. the production of melanin in melanocytes [Kaufmann et al., 1991; De Schepper et al., 2005], of osteopontin in osteoblasts [Li et al., 2009], of FGF-2, PDGF and midkine in Schwann cells [Mashour et al., 2001], or of collagen in fibroblasts [Yang et al., 2006].
Some NF1+/− cells display more variable sizes and shapes than their NF1+/+ counterparts, when they are cultured on surfaces with high stiffness such as Schwann cells [Rosenbaum et al., 2000], keratinocytes [Koivunen et al., 2000; Korkiamäki et al., 2002] or osteoclasts [Heervä et al., 2010; Stevenson et al., 2011]. Several findings demonstrate alterations in the cytoskeletal organization by NF1 haploinsufficiency. Visualization of individual cytoskeletal components has revealed irregular organization of the whole cytoskeletal system in NF1+/− keratinocytes [Koivunen et al., 2000]. Cytoskeletal actin abnormalities were observed in Nf1+/− astrocytes [Gutman et al., 2001]. An increased actin belt formation was shown in human derived cultured NF1+/− osteoclasts [Heervä et al., 2010; Stevenson et al., 2011]. In cultured NF1+/− melanocytes, an increased variation of dendrite formation was found [Kemkemer et al., 2002]. In these cells, increased noise in regulation of dendrite formation is an effect of NF1 haploinsufficiency.
NF1 encodes a very large 2,818 amino acid protein. Neurofibromin acts as a Ras-specific GTPase-activating protein (RasGAP) downregulating the biological activity, especially of K-Ras influencing the mitogen-activated Ras-MAPK pathway activity [Dasgupta et al., 2005a; McClatchey et al., 2007]. The RasGAP activity is mediated by a central portion, termed GAP-related domain. In search for functions of neurofibromin beyond the RasGAP activity, a number of interaction partners other than Ras have been identified [Welti et al., 2008], including kinesin-1, protein kinase A (PKA) and C (PKC), Syndecan, Caveolin-1 [Boyanapalli et al., 2006], glycerophospholipids [Welti et al., 2007] and the amyloid precursor protein. Very recently a direct binding between neurofibromin and SPRED1 was reported [McClatchey et al., 2012; Stowe et al., 2012]. This interaction seems to be very important for membrane localization of neurofibromin. Neurofibromin regulates the actin filament dynamics via the Rho-ROCK-LIMK2-Cofilin pathway [Boyanapalli et al., 2006]. Several additional pathways are regulated additionally by neurofibromin, such as mTOR and Akt [Dasgupta et al., 2005b; Lee et al., 2007; Banerjee et al., 2011]. The central GRD is flanked by a lipid-binding Sec14p-like domain (NF1-Sec) and a pleckstrin homology-like domain (NF1-PH) adjacent to and interacting with the NF1-Sec portion [D'Angelo et al., 2006; Welti et al., 2008, 2011]. In addition, a physical interaction between neurofibromin and focal adhesion kinase (FAK) has been reported, suggesting an involvement of neurofibromin in cell adhesion [Kweh et al., 2009]. FAK is found in focal adhesions, complex protein clusters linking the intracellular actin cytoskeleton to the extracellular environment via adhesion receptors in the membrane of cells. Focal adhesions and associated proteins such as FAK are supposed to be key players in the process of mechanotransduction, the translation of mechanical cues of the environment into biochemical intracellular signals.
Here the function of neurofibromin in the sensoric of extracellular topographies was investigated in cultured fibroblasts. Cells sense the topography of their environment and interact with native topographical structures as given by collagen bundles in many ways. For example, local variations in geometry can be transduced into biochemical signals that result in various cell responses. The underlying molecular mechanisms are often described as mechanotransduction [Vogel, 2006]. Various in vitro observations of responses across cell type, feature size, feature geometry, and the physical properties of the substrate material, including substrate stiffness [Yim and Leong, 2005; Dalby et al., 2007; Loesberg et al., 2007; Biela et al., 2009; Janmey et al., 2011] have demonstrated the importance of mechanotransduction.
One method to measure the effects of such physical signals on cells is the use of surfaces with well-defined surface topographies [Dalby, 2005; Vogel and Sheetz, 2006; Lim and Donahue, 2007]. Naturally occurring topographic structures within the extracellular matrix present topographical cues that influence cellular behavior. The size of the topographical structures can be either in the nanometer-range or in the range of micrometer. Some extracellular matrix proteins such as collagen molecules, for example, exhibit abundant, nanometer-scale structures, and bundles of these fibers can be micrometers in size. Also the basement membranes of many tissues exhibit rich topographies [Orr et al., 2006].
Micro- and nanofabrication techniques such as soft lithography enable the fabrication of substrates that mimic the structure and length scale of native topographies in 2-dimensions [Lim and Donahue, 2007; Lim et al., 2007], allowing for detailed in vitro studies. One well-studied example of cell responses to surface topographies on micro-manufactured substrates is the so-called contact guidance [Dalby, 2005]. In contact guidance, cells respond to topography structures on sub-micrometer scale by adapting their cell morphology with respect to elongated surface structures [Jungbauer et al., 2004]. Studies revealed a cell-type specific sensitivity in the contact guidance behavior of human endothelial cells, smooth muscle cells and fibroblasts [Biela et al., 2009]. Surface topographies can also alter the gene expression profiles of various cell types [Lim et al., 2007]. It is suggested that surfaces could be utilized as a signaling modality for directing differentiation, e.g. of human mesenchymal stem cells. Cultured on surfaces with a specific topography, these stem cells preferentially differentiated into neuronal lineages suggesting the potential of structured substrates to induce selective stem cell differentiation [Kurpinski et al., 2006]. The cellular mechanism of sensoric of extracellular topograhies is not completely understood.
We investigated the cell response in vitro to topographic structured surfaces of NF1 haploinsufficient fibroblasts. Since adhesions clusters and cytoskeleton structures are thought to be key components of the contact guidance response, we specifically investigated if NF1 haploinsufficiency can alter this type of cell response.
In a pilot study, NF1+/− and aged fibroblasts were cultured on surfaces with different elongated features (grooves), and first evidences were found for an altered orientation response of these cells [Kaufmann et al., 2011]. Here, in a detailed study including the use of neurofibromin pathyway inhibitors, we show that indeed the mechanosensitivity to specific topographies is altered in fibroblasts from various NF1 patients, indicating a new function of neurofibromin in the cellular sensoric of extracellular topograhies.
Materials and Methods
Cell Culture Substrates
Master wafers were produced by photolithography as moulds for transferring the topography pattern to biocompatible polydimethylsiloxane (PDMS) (Sylgard 184; Dow Corning, Mich., USA) substrates [Jungbauer et al., 2004] (fig. 1). They contained several rectangles with parallel grooves of different heights (h: 100 and 200 nm) and groove widths (d: 2, 3, 5, and 10 μm). The areas between the rectangles not containing grooves were used as control surfaces. The PDMS substrates were produced by mixing the pre-polymer with the curing agent with a ratio of 10:1 and cured on the master wafer at 65°C for 24 h. To increase surface wettability and allowing for cell adhesion, before cell experiments, the substrates were treated either with oxygen plasma (200 W, 1 mbar, Tepla RIE, Germany) for 8 s and 4 h with fetal bovine serum (FBS) or by coating 2 h with Poly-L-Lysin and 4 h with FBS. PDMS substrates were sterilized with 70% ethanol, washed 3× by PBS and finally used in the experiments. The surface topography of the substrates was verified by atomic force microscopy and scanning electron microscopy [Kemkemer et al., 2006].
Fig. 1.
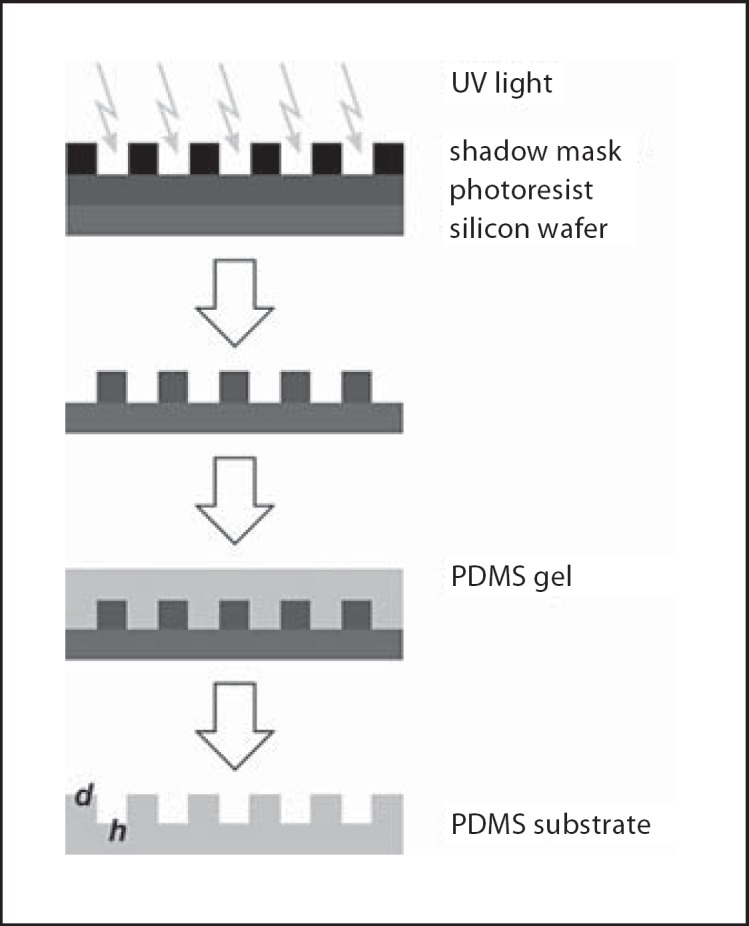
Principle of soft lithography and generation of microstructured PDMS substrates for cell cultures. Grooves: d = width, h = height.
Cell Culture
The processing of tissue and the preparation of the human fibroblasts was performed as described by Griesser et al. [1995]. Biopsies from 9 healthy male and female donors (K5–K23, aged 3–55 years) were obtained from the prepuce or the skin of the upper arm (table 1). The skin samples of NF1 patients were isolated from excisions of dermal neurofibromas using the adhering normal skin outside of the tumors. All NF1 patients (aged 9–52 years) were clinically examined by the NF clinic Ulm or by one of the authors (D.K.) and met the clinical criteria for neurofibromatosis 1. One patient (NF180) was suggested for cutaneous NF1 mosaicism. In 5 of 8, the NF1 germ line mutation is characterized (table 1). The research carried out was in compliance with the Helsinki Declaration (Ethikkommission Universität Ulm, A 185/09). The fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS at 37°C and 5% CO2. Cells were seeded on the coated PDMS substrates in regular culture media in a density of 15,000 cells/cm2. After 24 or 48 h, at least 16 micrographs at randomly chosen positions were taken for each surface topography by a AxioCam Mrm CCD camera attached to an AxioVert 200 microscope (Zeiss, Oberkochen, Germay) equipped with a 10× phase contrast objective.
Table 1.
Origin of the cultured cells
| Name | Age, years | Gender | NF1 | Passages | NF1 mutation |
|---|---|---|---|---|---|
| Controls | |||||
| K17 | 3 | male | NF1+/+ | 5 | – |
| K14 | 11 | male | NF1+/+ | 6 | – |
| K9 | 22 | female | NF1+/+ | 8 | |
| K8 | 23 | male | NF1+/+ | 6 | – |
| K20 | 24 | female | NF1+/+ | 7 | – |
| K24 | 29 | female | NF1+/+ | 8 | |
| K5 | 31 | male | NF1+/+ | 4 | – |
| K19 | 40 | male | NF1+/+ | 8 | – |
| K23 | 55 | female | NF1+/+ | 7 | – |
| NF1 patients | |||||
| NF329 | 9 | female | NF1+/− | 5 | c.6085-1G>A |
| NF82c | 18 | male | NF1+/− | 4 | unknown |
| NF183 | 26 | female | NF1+/− | 7 | 2972insT |
| NF82b | 31 | male | NF1+/− | 4 | unknown |
| NF196 | 32 | female | NF1+/− | 8 | 655insTT |
| NF324 | 39 | male | NF1+/− | 4 | unknown |
| NF213 | 41 | male | NF1+/− | 7 | 2590insTATA |
| NF84a | 52 | female | NF1+/− | 8 | 887delA |
Image and Data Analysis
Microscopy images of the cells were analyzed by ImageJ software (http://rsb.info.nih.gov/ij/). First, the contours of the cells were marked, and an ellipse was fitted to the outline of each cell. The orientation angle of each ellipse, as well as the angle of the groove microstructures (β), was determined in order to calculate the cell orientation angle (α) (fig. 2). This angle gives the orientation of the cell's long axis relative to the direction of the grooves. To characterize the mean cell orientation, the order parameter (S = <cos(2α)>) was calculated [Kemkemer et al., 2006]. Fibroblasts are randomly orientated for S = 0 and perfectly aligned along the grooves for S = 1. At least 100 cells per surface condition were evaluated (N).
Fig. 2.
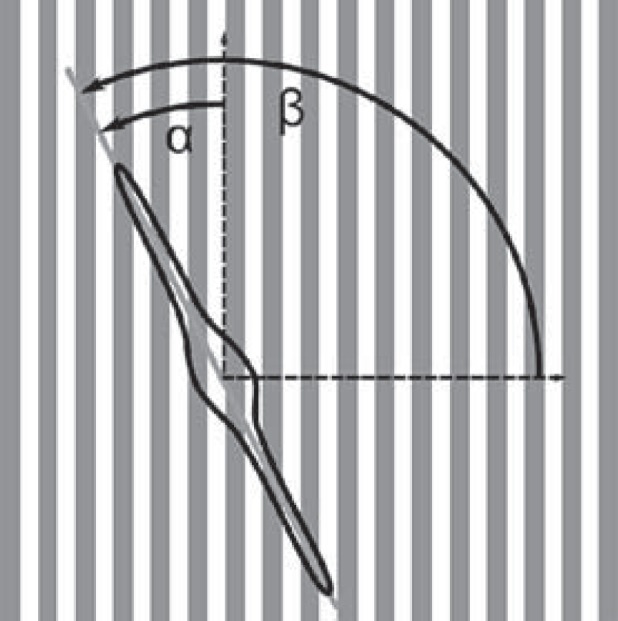
Cell orientation in respect to the orientation of grooves. The angle α was measured. To characterize the mean cell orientation, the order parameter S = <cos(2α)> was calculated: random orientation: S = 0, perfect orientation along the grooves: S = 1.
Neurofibromin Pathway Inhibitors
NF1+/− fibroblasts (NF239) were treated for 2 days with the following inhibitors (all derived from Calbiochem, Darmstadt, Germany) influencing the neurofibromin pathway: FTI-277 (30 μm, HRAS Inhibitor) [Ammoun et al., 2008], PD98059 (25 μm, MAPK-Inhibitor) and Y27632 (10 μm, ROCK-Inhibitor) [Huang et al., 2004], and Rapamycin (5 μm, mTOR-inhibitor) [Dasgupta et al., 2005b]. The Rac-Inhibitor NSC23766 (50 μm), [Xu et al., 2009] derived from Toris Bioscience (Bristol, UK). The doses of the inhibitors are related to the doses described in the literature cited above.
Statistical Analysis
Statistical analysis was carried out by Origin software (Microcal; OriginLab Corporation, Northampton, Mass., USA). The mean values and standard errors (SEM) were compared with each other. Statistical analysis was performed with the t-test while p < 0.05 was defined as statistically significant.
Results
Orientation of Control Fibroblasts Depends on Height and Width of the Submicron Topographies
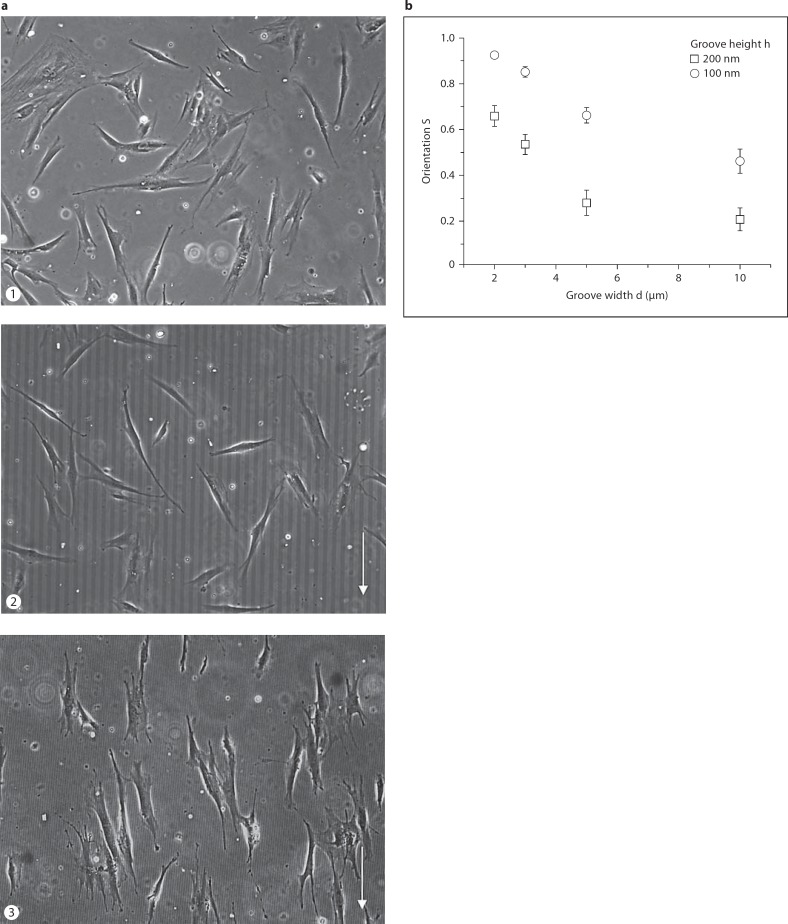
The orientation of cells on extracellular surface topographies depends on the groove height and width of the microgrooved structures. First, we tested which topography results in a high degree of orientation for the adhesive cells. Primary control fibroblasts (K14) were cultured on PDMS substrates with different groove heights (h = 100 and 200 nm) and widths (d = 2, 3, 5 and 10 μm). On a flat PDMS surface, the cells are randomly aligned with no preferred orientation (fig. 3a). Cultured on groove topographies, they aligned their cell body preferably in the direction of the grooves. For a given groove height (h), the orientation decreases with increasing groove width (d) (fig. 3b). The alignment of cells along the grooves is highest for the width of d = 2 μm and smallest for d = 10 μm. The order parameter S is higher for cells on substrates with h = 200 nm than for those on substrates with h = 100 nm for all 4 grooves widths d. The control fibroblasts are almost perfectly aligned along the groove direction for d = 2 μm and h = 200 nm. The small standard deviation of the order parameter S indicates a small variation in cell orientation under these conditions.
Fig. 3.
Highest orientation of control fibroblasts was found along the grooves in PDMS substrates with height of 200 nm and width of 2 μm. a Phase contrast microscopy images of human fibroblasts of a control (K14) cultured for 2 days on PDMS substrates (1) without grooves, (2) with grooves of d = 10 μm and h = 200 nm and (3) with d = 2 μm (h = 200 nm). Arrow: orientation of the grooves. b The average orientation (S = <cos(2α)>) of the control fibroblasts (K14) was determined by measuring the angle α. The cells were cultured on substrates with groove heights h = 200 nm (circles) and h = 100 nm (squares) and groove widths d of 2, 3, 5, and 10 μm; N = 100–145.
Differences in Orientation between Control and NF1 Fibroblasts Are Time and Topography Dependent
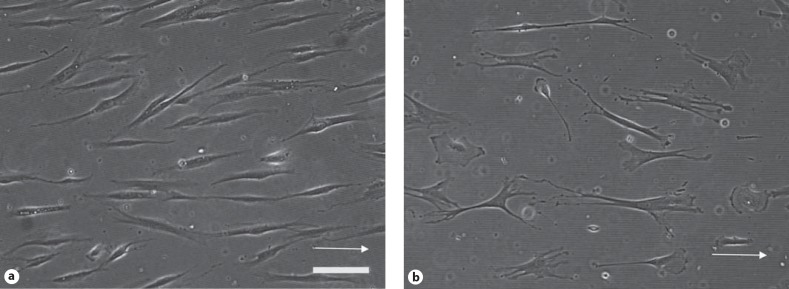
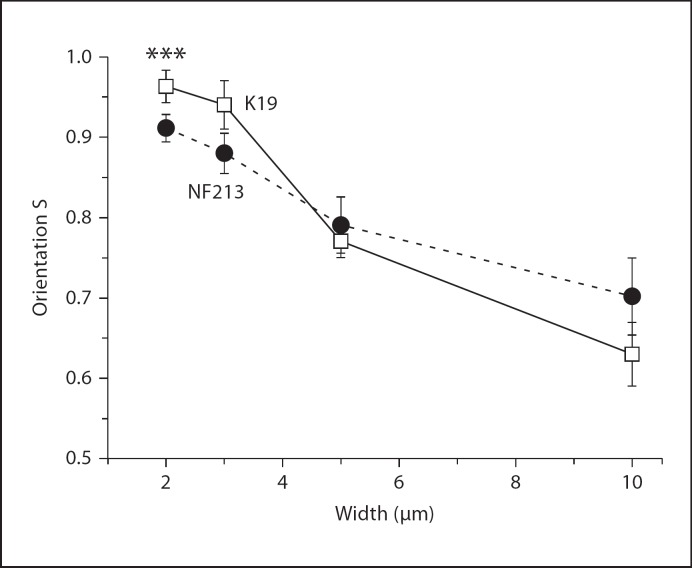
To investigate if there are differences between control and NF1 cells depends on the width of the grooves, 3 age-matched cell pairs were cultured on grooves with 2, 3, 5 and 10 μm for 2 days. The control and NF1 fibroblasts differ in shape (fig. 4). NF1 cells aligned along the groove direction in a similar way as controll cells; however, differences in the order parameter S were most obvious (p < 0.001) in cells cultured on grooves of 2 μm width as demonstrated for the pair K19/NF213 (fig. 5). The order parameter S is reduced for NF1 cells at these conditions. On grooves with a width of 10 μm, the differences in S between the cells were diminished; NF1 and control cells were in average orientated to a similar degree. Analogous results were obtained for the 2 additional pairs (K8/NF239 and K8/NF239, data not shown).
Fig. 4.
Fibroblasts of a control and NF1 differ in shape. The age-matched cell pair was cultured for 2 days on PDMS substrates (grooves d = 2 μm, h = 200 nm). Phase contrast microscopy images of (a) fibroblasts derived from the skin of control (K14) or (b) a NF1 patient (NF82b). Arrow: orientation of the grooves. Scale bar: 50 μm.
Fig. 5.
Higher relative orientation of control fibroblasts as NF1+/− fibroblasts on grooves with a width of 2μm. Age-matched pairs of control und NF1 fibroblasts (K19/NF213) were cultured for 2 days on substrates with grooves with different widths (h = 200 nm, d = 2, 3, 5, and 10 μm). The cellular orientation S (S = <cos(2α)>) was determined between control and NF1 fibroblasts by measuring the angle α. N = 141–201; P(C) ≠ S(NF1). ***p < 0.001.
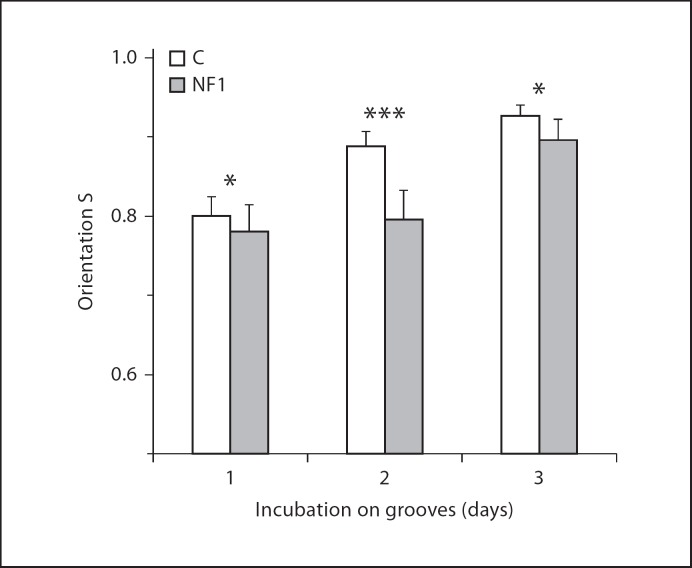
The orientation of cells on submicron topographies requires the spreading of the cells and the adjustment of structural components like the cytoskeleton. In addition, fibroblasts synthesize extracellular matrix which may interfere with the effects of the microgrooved topographies on orientation of cells. Therefore, we tested the cell alignment at different time points after cell seeding. Results for control fibroblasts (K19) grown on substrates allowing for a high degree of orientation (d = 2 μm, h = 200 nm) are given for day 1, 2 and 3 after seeding. At all 3 time points, the fibroblasts align along the grooves. However, the orientation increases in time (fig. 6).
Fig. 6.
Control and NF1+/− fibroblasts differ in orientation in time. The cell pair (K19 and NF213) was cultured for 1, 2 and 3 days on grooves (d = 2 μm, h = 200 nm). The average orientation was determined. N = 138–348. ***p < 0.001, *p < 0.05.
Then we tested age-matched NF1 fibroblasts (NF291) for their orientation in time. Their orientation parameter (S) increases in time, but was lower at all tested time points (fig. 6). The highest difference in S between the cells of the control and the NF1 patient was found at day 2 (p < 0.001). In order to further test difference in orientation of cells from several healthy and NF1 donors, we used the experimental condition that proved to show the largest differences in the response of NF1 and control cells.
NF1 Fibroblasts Are Less Sensitive to a Specific Submicron Topography than Control Fibroblasts
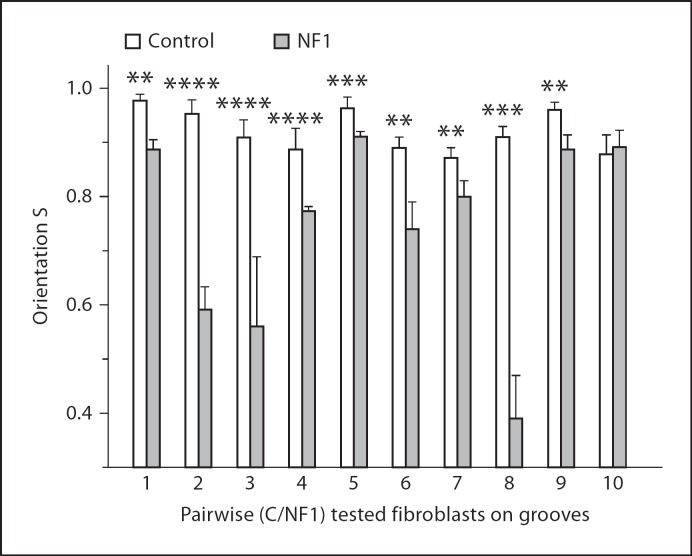
Orientation of aged-matched fibroblast cultures of 9 healthy donors and 8 NF1 patients was measured in 10 independent experiments using the protocol given above. As shown in figure 7, in 9 of 10 experiments the fibroblasts from healthy donors show a relative higher S than the fibroblasts of NF1 patients. In summary, the difference in orientation between control and NF1 fibroblasts is significant (p = 0.0078). NF1+/− fibroblasts are less sensitive to specific extracellular microgrooved topography than aged-matched fibroblast of healthy donors.
Fig. 7.
NF1+/− fibrobasts are less orientated on defined topographies than NF1+/+ fibroblasts. Cells were cultured for 2 days on structured PDMS substrates (d = 2 μm, h = 200 nm). The pairs are aged and passage (p) matched. Pair 1: K20/NF183 (p7/p7); pair 2: K8/NF329 (p6/p5); pair 3: K17/NF329 (p5/p5); pair 4: K19/NF213 (p5/p5); pair 5: K19/NF213 (p7/p8); pair 6: K5/NF82c (p5/5); pair 7:K8/NF324 (p5/p5); pair 8: K14/NF82b (p5/p5); pair 9: K23/NF84a (p7/p8); pair 10: K24/NF196 (p8/p8); pair 11:K9/NF180 (p8/p8). N = 110–436; p for S(C) ≠ S(NF1). ****p < 0.0001, ***p < 0.001, **p < 0.01.
The Reduced Topographic Sensitivity in NF1+/− Cells Can Be Partly Rescued by Inhibitors of Pathways Dysregulated by NF1 Haploinsufficiency
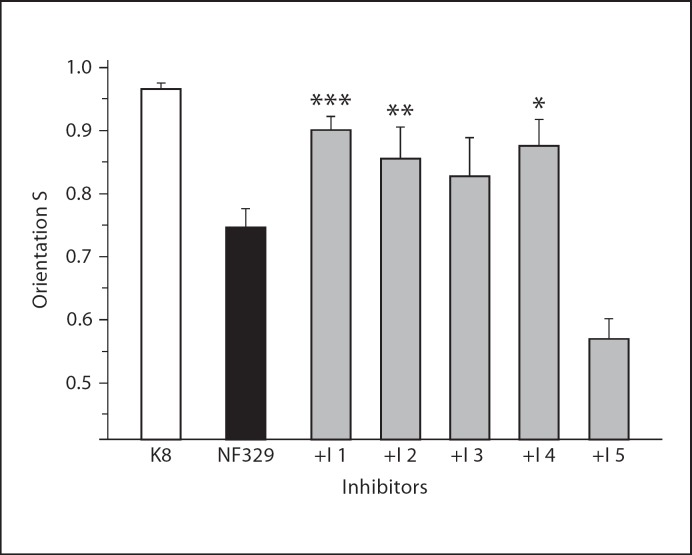
The reduced amount of neurofibromin in the NF1+/− cells results in an increased activity of proteins in several pathways, such as Ras, MAPK, Rac, mTOR, and ROCK. A reduction of their activity in NF1+/− cells could influence the tested cell biological parameter, their orientation on nanometer microgrooved surface. In order to test this, cells were treated for 2 days with neurofibromin pathway-related inhibitors. Four out of 5 of these inhibitors ameliorate the orientation of the NF1 cells (NF329). Inhibition of HRAS activity by incubation with a farnesytransferase inhibitor (FTI-277) significantly increases the orientation of NF1+/− fibroblasts (p < 0.001) (fig. 8). Also the inhibition of the cellular Rac activity by the inhibitor NSC23766 increases the orientation of the NF1+/− cells (p < 0.01). In contrast, inhibition of ROCK activity by incubation with Y27632 results in a loss of cellular orientation on the grooves. This experiment illustrates that the reduced sensitivity of NF1+/− fibroblasts can be rescued partly by inhibitors of the neurofibromin pathyway.
Fig. 8.
Inhibitors of pathways dysregulated by NF1 haploinsufficiency ameliorate the mean orientation (S) in NF1+/− fibroblast. NF329 fibroblasts were treated for 2 days with inhibitors (I) of neurofibromin regulated pathways. I 1: Ras (30 μm FTI-277); I 2: Rac (50 μm NSC23766); I 3: MAPK (25 μm PD98059); I 4: mTOR (5 μm Rapamycin); I 5: ROCK (10 μm Y27632). K8: fibroblasts of an age matched control. S = <cos(2α)>: orientation of the cells on the grooves (h = 200 nm, d = 2 μm). N = 122–173 cells; p: S(NF1 untreated) ≠ S(NF1 treated). ***p < 0.001, **p < 0.01, *p < 0.05.
Discussion
In our study, we present first evidence for a reduced contact guidance reaction of NF1+/− fibroblasts if cultured on defined extracellular submicron topographies. The finely tuned interaction between cells and their physical surroundings is crucial for the regulation of many important cellular functions. The in- and outward signaling can be disturbed by mutations in genes regulating cellular mechanotransduction [Jaalouk and Lammerding, 2009]. The cellular mechanisms for sensing geometries or topographies share common features with the mechanisms for sensing and transducing forces and mechanically induced deformations into biochemical signals, finally leading to biological responses [Vogel and Sheetz, 2006]. Therefore, one may assume a broad relevance of our observation.
In our experiments, an orientation response to very small topography with a height of 100 nm was found in cultured human control fibroblasts. Others have reported a topographic sensitivity of cultured fibroblasts to grooves of 35 nm height [Loesberg et al., 2007]. We found similar sensitivities also in other cells types [Kemkemer et al., 2006], especially in cultured melanocytes. These cells react on topographies with a height of 25 nm [Jungbauer et al., 2004]. The degree of alignment depends on this parameter and the lateral spacing of the grooves, as demonstrated again in cultured control melanoycytes [Kemkemer et al., 2006]. Our study does not prove that NF1 alters such a lower topography threshold to which cells respond, but the overall observation of reduced sensitivity may suggest that.
In our study, fibroblasts from healthy donors were more sensitive to specific submicron surfaces than cells taken from different NF1 patients. Thus, we suggest that NF1+/− fibroblasts show a partial ‘blindness’ in their mechanosensoric ability to respond to topographies in their environment. Since many details about cellular mechanosensoric responses to geometry and topography are not understood, we can only speculate about the underlying reasons for this altered behavior of the NF1+/− cells. It has been proposed that by aligning along grooved surfaces cells minimize the distortion of their cytoskeleton that is induced by the topography [Hoffman-Kim et al., 2010]. This hypothesis is supported by the observation of actin stress fiber alignment along the grooves [Teixeira et al., 2003; Gerecht et al., 2007; Kaufmann et al., 2011]. Moreover, experiments by others demonstrated that actin filament dynamics is affected by a reduced neurofibromin dose in human fibrosarcoma cell line via changes in the Rho-ROCK-LIMK2-Cofilin pathway [Ozawa et al., 2005]. The increased actin belt formation in human-derived cultured NF1+/− osteoclasts is also a consequence of the hyperactivity RhoGTPase pathway [Stevenson et al., 2011]. These observations suggest that a NF1 haploinsufficiency might cause an altered organization and assembly of cytoskeletal proteins, which may affect cell mechanical properties of the cells. Assuming that the minimization of mechanically induced stress or deformation is the key mechanism for cell alignment along directed topographical cues, changes in the cytoskeleton may cause the altered sensitivity of NF1+/− cells. This idea is supported by the observation of cytoskeletal abnormalities in several NF1+/− cells, e.g. in cultured melanocytes [Kaufmann et al., 1991; Kemkemer et al., 2002; Jungbauer et al., 2004] and keratinocytes [Koivunen et al., 2000]. The reduced number of cytokeratin bundles in differentiating cultured NF1+/− keratinocytes indicates a function of neurofibromin in the organization of cytoskeleton during the formation of cellular contacts [Koivunen et al., 2000]. In Nf1+/− astrocytes, decreased cell attachment and increased cell motility were contributed to actin cytoskeletal abnormalities [Gutmann et al., 2001].
Other suggested key players in the transduction of signals from the extracellular matrix to the cell inside are integrins and focal adhesion, complex protein clusters linking the intracellular actin cytoskeleton to the cell environment via adhesion receptors in the membrane of cells. Therefore, the physical interaction between neurofibromin and FAK observed in mouse fibroblasts [Kweh et al., 2009] might suggest an involvement of neurofibromin in mechanotransduction pathways. Since focal adhesions and associated proteins such as the FAK are supposed to be essential elements in the process of mechanotransduction, these results may support our hypothesis that the NF1 gene has a functional role in the reception of mechanical signals.
It is unclear what significance our findings in cultured NF1+/− fibroblasts have for in vivo situations. However, the ability of cells to sense and respond to topography is of great importance for maintaining cell and tissue integrity and functionality [Wang and Li, 2010]. Therefore, we propose that additional cell types should be investigated in detail and in other mechanotransduction tests to reveal if blindness in mechanosensoric pathways is a general phenomenon of NF1+/− cells. In addition, the effects of the complete loss of neurofibromin (NF1−/−) on mechanosensoric and mechanotransdcution should be investigated in several cell types. Although still speculative, an altered sensitivity to topography or mechanical signals of the cell environment in NF1+/− cells may contribute to the pathogenesis of phenotypes in NF1. One example may be a non-tumor-phenotype, the alterations found in the vascular system of Nf1+/− mice, especially after vascular injury corresponding to findings in NF1 patients [Lasater et al., 2008]. Another speculative example may be the altered behavior of the fibroblasts in the cutaneous neurofibromas [Jouhilahti et al., 2011]. In addition, it will be interesting to compare the interpersonal variation in contact guidance of NF1+/− fibroblasts and clinical NF1 parameters, e.g. the tumor load especially concerning dermal neurofibromas or the degree of learning disabilities of the patients. NF1 is one of the genetic diseases related to the Ras pathway called Rasopathies, which share some symptoms [Zenker, 2011]. In this view, an investigation on mechanosensoric functions of cells from patients with other diseases of the Ras pathway will be interesting. In addition, more precise studies will be necessary to characterize the specific neurofibromin dependent pathway involved in the regulation of topographic sensoric and mechanotransduction.
Conclusions
The use of artificial surfaces with small topographies as substrates for in vitro culture helped to detect a reduced mechanosensoric response of NF1+/− fibroblasts in the alignment to such guiding cues. Therefore, we propose a new functional role of neurofibromin in the process of cellular mechanosensoric of topographies and in mechanotransduction. This function may help to further reveal non-tumor clinical phenotypes in NF1.
Acknowledgements
We thank Christine Mollenhauer, Heidrun Götz and Monika Dodrimond-Lattke for technical assistance and Josef Högel for statistical investigations. D.K. is supported by a BMBF grant.
References
- Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Crouse NR, Emnett RJ, Gianino SM, Gutmann DH. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci USA. 2011;108:15996–16001. doi: 10.1073/pnas.1019012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biela S, Su Y, Spatz JP, Kemkemer R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano-micro range. Acta Biomater. 2009;5:2460–2466. doi: 10.1016/j.actbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Boyanapalli M, Lahoud OB, Messiaen L, Kim B, Anderle de Sylor MS, et al. Neurofibromin binds to caveolin-1 and regulates ras, FAK and Akt. Biochem Biophys Res Commun. 2006;340(4):1200–1208. doi: 10.1016/j.bbrc.2005.12.129. [DOI] [PubMed] [Google Scholar]
- Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborates paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16:1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- Dalby MJ. Topographically induced direct cell mechanotransduction. Med Eng Phys. 2005;27:730–742. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Herzyk P, Agheli H, Sutherland S, Wilkinson CD. Group analysis of regulation of fibroblast genome on low-adhesion nanostructures. Biomaterials. 2007;28:1761–1769. doi: 10.1016/j.biomaterials.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005a;65:236–245. [PubMed] [Google Scholar]
- Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005b;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- De Schepper S, Boucneau J, Lambert J, Messiaen L, Naeyaert JM. Pigment cell-related manifestations in neurofibromatosis type 1: an overview. Pigment Cell Res. 2005;18:13–24. doi: 10.1111/j.1600-0749.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- Ferner RE. Neurofibromatosis 1. Eur J Hum Genet. 2007;15:131–138. doi: 10.1038/sj.ejhg.5201676. [DOI] [PubMed] [Google Scholar]
- Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis Type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus. 2010;28:E8. doi: 10.3171/2009.11.FOCUS09221. [DOI] [PubMed] [Google Scholar]
- Griesser J, Kaufmann D, Eisenbarth I, Bäuerle C, Krone W. Ras-GTP regulation is not altered in cultured melanocytes with reduced levels of neurofibromin derived from patients with neurofibromatosis 1 (NF1) Biol Chem Hoppe Seyler. 1995;376:91–101. doi: 10.1515/bchm3.1995.376.2.91. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Wu YL, Hedrick NM, Zhu Y, Guha A, Parada LF. Heterozygosity for the neurofibromatosis 1 (NF1) tumor suppressor results in abnormalities in cell attachment, spreading and motility in astrocytes. Hum Mol Genet. 2001;10:3009–3016. doi: 10.1093/hmg/10.26.3009. [DOI] [PubMed] [Google Scholar]
- Heervä E, Alanne MH, Peltonen S, Kuorilehto T, Hentunen T, et al. Osteoclasts in neurofibromatosis type 1 display enhanced resorption capacity, aberrant morphology, and resistance to serum deprivation. Bone. 2010;47:583–590. doi: 10.1016/j.bone.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Rangwala F, Fulkerson PC, Ling B, Reed E, et al. Role of TC21/R-Ras2 in enhanced migration of neurofibromindeficient Schwann cells. Oncogene. 2004;23:368–378. doi: 10.1038/sj.onc.1207075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Yang FC, Travers JB, Wenning MJ, Hiatt K, et al. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J Exp Med. 2000;191:181–188. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhilahti EM, Peltonen S, Heape AM, Peltonen J. The pathoetiology of neurofibromatosis 1. Am J Pathol. 2011;178:1932–1939. doi: 10.1016/j.ajpath.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbauer S, Kemkemer R, Gruler H, Kaufmann D, Spatz JP. Cell shape normalization, dendrite orientation, and melanin production of normal and genetically altered (haploinsufficient NF1)-melanocytes by microstructured substrate interactions. Chemphyschem. 2004;5:85–92. doi: 10.1002/cphc.200300868. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, Wiandt S, Veser J, Krone W. Increased melanogenesis in cultured epidermal melanocytes from patients with neurofibromatosis 1 (NF1) Hum Genet. 1991;87:144–150. doi: 10.1007/BF00204170. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, Su Y, Biela S, Jungbauer S, Spatz JP, Kemkemer R. Soft lithography detects partial mechano-sensoric blindness to micrometre topography in cultured aged and diseased cells. IJMR. 2011;102:896–902. [Google Scholar]
- Kemkemer R, Schrank S, Vogel W, Gruler H, Kaufmann D. Increased noise as an effect of haploinsufficiency of the tumor-suppressor gene neurofibromatosis type 1 in vitro. Proc Natl Acad Sci USA. 2002;99:13783–13788. doi: 10.1073/pnas.212386999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemkemer R, Jungbauer S, Kaufmann D, Gruler H. Cell orientation by a microgrooved substrate can be predicted by automatic control theory. Biophys J. 2006;90:4701–4711. doi: 10.1529/biophysj.105.067967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf WF, Yang FC, Chen S, White H, Bessler W, et al. K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/- mast cells. J Immunol. 2007;178:2527–2534. doi: 10.4049/jimmunol.178.4.2527. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Ylä-Outinen H, Korkiamäki T, Karvonen SL, Pöyhönen M, et al. New function for NF1 tumor suppressor. J Invest Dermatol. 2000;114:473–479. doi: 10.1046/j.1523-1747.2000.00882.x. [DOI] [PubMed] [Google Scholar]
- Korkiamäki T, Ylä-Outinen H, Koivunen J, Karvonen SL, Peltonen J. Altered calcium mediated cell signaling in keratinocytes cultures from patients with neurofibromatosis type 1. Am J Pathol. 2002;160:1981–1990. doi: 10.1016/S0002-9440(10)61148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuorilehto T, Pöyhönen M, Bloigu R, Heikkinen J, Väänänen K, Peltonen J. Decreased bone mineral density and content in neurofibromatosis type 1: lowest local values are located in the load-carrying parts of the body. Osteoporos Int. 2005;16:928–936. doi: 10.1007/s00198-004-1801-4. [DOI] [PubMed] [Google Scholar]
- Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Mol Carcinog. 2009;48:1005–1017. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert M, Kappler M, Mautner VF, Lammert K, Storkel S, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161–1166. doi: 10.1007/s00198-005-1940-2. [DOI] [PubMed] [Google Scholar]
- Lasater EA, Bessler WK, Mead LE, Horn WE, Clapp DW, et al. Nf1+/- mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–2344. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Stephenson DA. Recent developments in neurofibromatosis type 1. Curr Opin Neurol. 2007;20:135–141. doi: 10.1097/WCO.0b013e3280895da8. [DOI] [PubMed] [Google Scholar]
- Li H, Liu Y, Zhang Q, Jing Y, Chen S, et al. Ras dependent paracrine secretion of osteopontin by Nf1+/- osteoblasts promote osteoclast activation in a neurofibromatosis type I murine model. Pediatr Res. 2009;65:613–618. doi: 10.1203/PDR.0b013e3181a1c607. [DOI] [PubMed] [Google Scholar]
- Lin YL, Lei YT, Hong CJ, Hsueh YP. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- Lim JY, Dreiss AD, Zhou Z, Hansen JC, Siedlecki CA, et al. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787–1797. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Loesberg WA, te Riet J, van Delft FC, Schön P, Figdor CG, et al. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28:3944–3951. doi: 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Ratner N, Khan GA, Wang HL, Martuza RL, Kurtz A. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- McClatchey AI. Neurofibromatosis. Annu Rev Pathol. 2007;2:191–216. doi: 10.1146/annurev.pathol.2.010506.091940. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Cichowski K. SPRED proteins provide a NF-ty link to Ras suppression. Genes Dev. 2012;26:1515–1519. doi: 10.1101/gad.197434.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, et al. Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/- mast cells. Blood. 2008;112:4646–4654. doi: 10.1182/blood-2008-04-155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Jacks T. Thinking beyond the tumor cell: Nf1 haploinsufficiency in the tumor environment. Cancer Cell. 2002;1:408–410. doi: 10.1016/s1535-6108(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Araki N, Yunoue S, Tokuo H, Feng L, et al. The neurofibromatosis type 1 gene product neurofibromin enhances cell motility by regulating actin filament dynamics via the Rho-ROCK-LIMK2-cofilin pathway. J Biol Chem. 2005;280:39524–39533. doi: 10.1074/jbc.M503707200. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Lloyd AC. Neurofibroma development in NF1–insights into tumour initiation. Trends Cell Biol. 2009;19:395–403. doi: 10.1016/j.tcb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Pemov A, Park C, Reilly KM, Stewart DR. Evidence of perturbations of cell cycle and DNA repair pathways as a consequence of human and murine NF1-haploinsufficiency. BMC Genomics. 2010;11:194. doi: 10.1186/1471-2164-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981;305:1617–1627. doi: 10.1056/NEJM198112313052704. [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Rosenbaum C, Winner U, Müller HW, Lenard HG, Hanemann CO. Long-term culture and characterization of human neurofibroma-derived Schwann cells. J Neurosci Res. 2000;61:524–532. doi: 10.1002/1097-4547(20000901)61:5<524::AID-JNR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Shapira S, Barkan B, Friedman E, Kloog Y, Stein R. The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. Cell Death Differ. 2007;14:895–906. doi: 10.1038/sj.cdd.4402057. [DOI] [PubMed] [Google Scholar]
- Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70:51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116:157–164. doi: 10.1182/blood-2009-09-242875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Annu Rev Pathol. 2012;7:469–495. doi: 10.1146/annurev-pathol-011811-132441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DA, Yan J, He Y, Li H, Liu Y, et al. Multiple increased osteoclast functions in individuals with neurofibromatosis type 1. Am J Med Genet A. 2011;155A:1050–1059. doi: 10.1002/ajmg.a.33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe IB, Mercado EL, Stowe TR, Bell EL, Oses-Prieto JA, et al. A shared molecular mechanism underlies the human rasopathies Legius syndrome and Neurofibromatosis-1. Genes Dev. 2012;26:1421–1426. doi: 10.1101/gad.190876.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang JH, Li B. Mechanics rules cell biology. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:16. doi: 10.1186/1758-2555-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti S, Fraterman S, D'Angelo I, Wilm M, Scheffzek K. The sec14 homology module of neurofibromin binds cellular glycerophospholipids: mass spectrometry and structure of a lipid complex. J Mol Biol. 2007;366:551–562. doi: 10.1016/j.jmb.2006.11.055. [DOI] [PubMed] [Google Scholar]
- Welti S, D'Angelo I, Scheffzek K. Structure and Function of Neurofibromin. In: Kaufmann D, editor. Neurofibromatoses, Monogr Hum Genet. Basel: Karger; 2008. pp. 113–128. [Google Scholar]
- Welti S, Kühn S, D'Angelo I, Brügger B, Kaufmann D, Scheffzek K. Structural and biochemical consequences of NF1 associated nontruncating mutations in the Sec14-PH module of neurofibromin. Hum Mutat. 2011;32:191–197. doi: 10.1002/humu.21405. [DOI] [PubMed] [Google Scholar]
- Wu M, Wallace MR, Muir D. Nf1 haploinsufficiency augments angiogenesis. Oncogene. 2006;25:2297–2303. doi: 10.1038/sj.onc.1209264. [DOI] [PubMed] [Google Scholar]
- Xu J, Ismat F, Wang T, Yang J, Epstein J. NF1 regulates a Ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–2156. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- Xu SW, Liu S, Eastwood M, Sonnylal S, Denton CP, et al. Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One. 2009;4:e7438. doi: 10.1371/journal.pone.0007438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Chen S, Clegg T, Li X, Morgan T, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/- and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Leong KW. Significance of synthetic nanostructures in dictating cellular response. Nanomedicine. 2005;1:10–21. doi: 10.1016/j.nano.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zenker M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr Opin Pediatr. 2011;23:443–451. doi: 10.1097/MOP.0b013e32834881dd. [DOI] [PubMed] [Google Scholar]