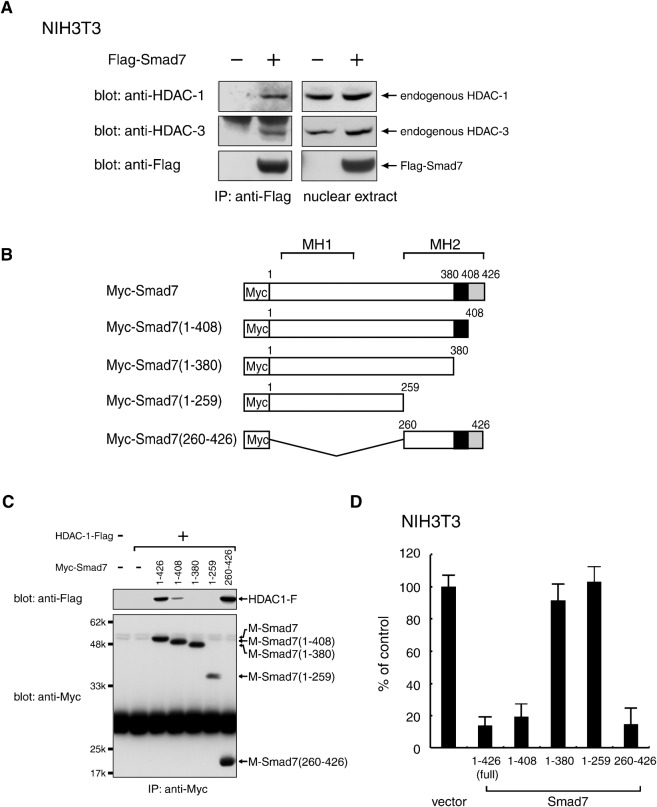
Fig. 1. Complex formation between Smad7 and specific HDAC proteins.
(A) Association of Smad7 with endogenous HDAC-1 and HDAC-3, respectively, in NIH 3T3 mouse fibroblast cells. Nuclear extracts from NIH 3T3 cells transfected with a vector containing control or Flag-Smad7 were incubated with an anti-Flag antibody to immunoprecipitate a complex containing Flag-Smad7. Immune complexes (left) and total nuclear extracts (right) were examined by Western blotting using α-HDAC-1, α-HDAC-3 and α-Flag antibodies. (B,C) Requirement of the 381–408 C-terminal region for the association of Smad7 with HDAC-1. A series of truncated Smad7 variants with an N-terminal Myc-tag (B) were expressed together with HDAC-1-Flag in 293T cells and immunoprecipitated with α-Myc. Immune complexes were analyzed by Western blotting using α-Flag and α-Myc. Black boxes indicate amino acids 381-408, which are essential for the interaction with HDAC-1 (C). (D) NIH3T3 cells transduced with wild-type Smad7 or each deletion mutant by a retrovirus vector were seeded in a 96-well plate (3×103 cells/well) and incubated for 72 h. [3H]-thymidine was loaded for the last 4 h and the radioactivity incorporated into chromosomal DNA was measured by a microplate scintillation counter. Data are shown as means with S.E. bars from triplicate assays.