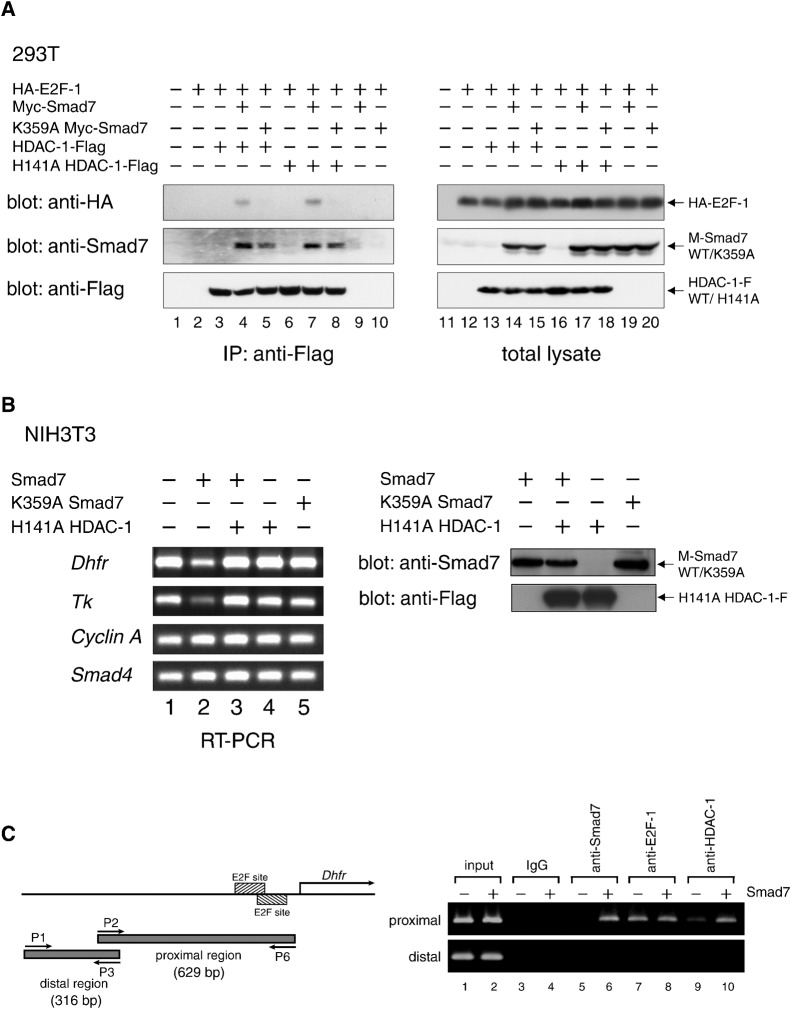
Fig. 5. Repression of E2F-regulated genes by Smad7-linked association between E2F-1 and HDAC-1.
(A) An in vivo complex of HDAC-1 and E2F-1 is mediated by Smad7 depending on Lys359. E2F1, Smad7, HDAC-1 and their variants, each with separate epitope tags, were expressed in 293T cells at the indicated combinations. Complexes containing HDAC-1-Flag or H141A HDAC-1-Flag were immunoprecipitated with α-Flag from total cell extracts. The immune complexes (left) and total lysates (right) were examined by Western blotting to detect E2F-1, Smad7 and HDAC-1 variants by using α-HA, α-Smad7 and α-Flag, respectively. (B) Smad7-induced repression of the Dhfr and Tk genes, both of which are regulated by E2F factors. (Left) Total RNA was prepared from NIH 3T3 cells infected with retro vectors for wild-type or K359A Smad7 and H141A HDAC-1, as shown, and cultured for 24 h. Complementary DNAs were synthesized from 1 µg RNA by reverse transcription with random primers. Equal aliquots from each reaction were used for PCR to amplify cDNA fragments of Dhfr (371 bp), Tk (411 bp), Cyclin A (459 bp) and Smad4 (358 bp), and subjected to agarose gel electrophoresis to detect the amplified products. (Right) Expression levels of wild-type/K359A Smad7 and H141A HDAC-1 were examined. Lysates from NIH3T3 cells infected as in (left) were analyzed by Western blotting using α-Smad7 or α-Flag antibody. (C) Association of Smad7 and HDAC-1 to the promoter region in the Dhfr gene that includes E2F-responsive sites. (Left) The promoter region of the mouse Dhfr gene is schematically represented. Hatched boxes denote E2F-responsive sites Fry et al., 1999). Lower arrows (P1 and P3; P2 and P6) indicate the positions of primer sets for PCR. The upper arrow (labeled as Dhfr) represents the major transcription start site Fry et al., 1999). A putative PCR fragment obtained from a control distal region (316 bp) and that from an E2F site-containing region (629 bp) proximal to the start site are shown as gray bars. (Right) Subregions in the promoter of the Dhfr gene were analyzed by chromatin immunoprecipitation assays by using control IgG and antibodies specific for Smad7, E2F-1 and HDAC-1, respectively. NIH 3T3 cells infected with control (−) or Smad7 vectors (+) were cultured in DMEM with 10% FBS for 14 h and harvested for assays. Genomic DNA fragments in the chromatin collected with each indicated antibody was used as a template for PCR. Products were subjected to electrophoresis to detect the fragments predicted in the left panel.