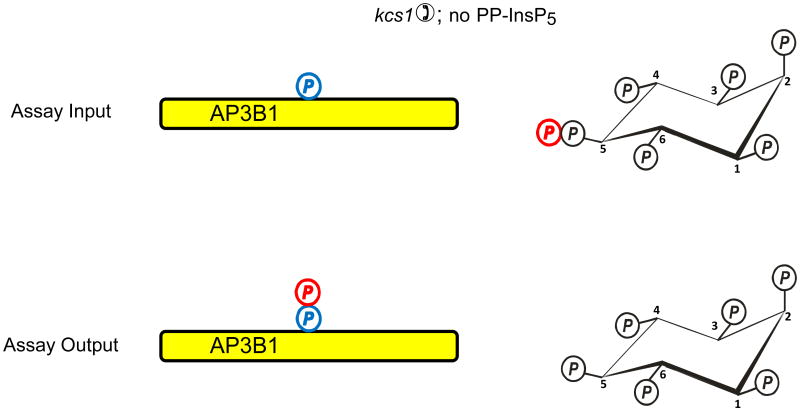
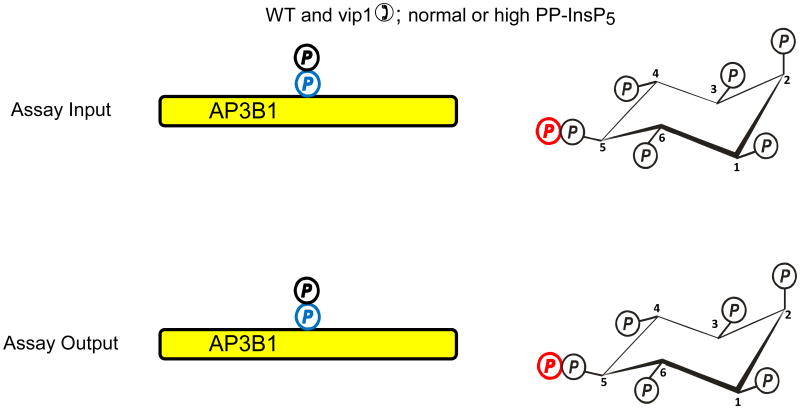
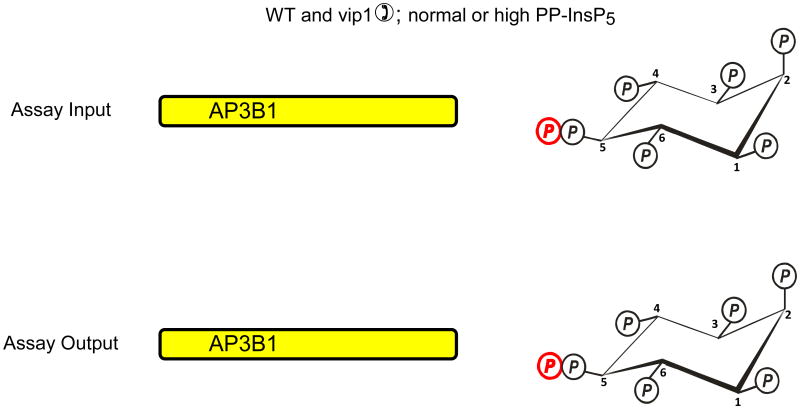
Fig. 2. Evidence that diphosphoinositol polyphosphates transphosphorylate proteins in intact cells?
The graphic depicts one particular site on AP3B1 (colored blue) that, in vivo, casein kinase II can mono-phosphorylate, thereby priming it to be transphosphorylated by PP-InsP5 (AP3B1 has other potential phosphorylation sites (Azevedo et al., 2009) that are not illustrated here). Panel a illustrates that Azevedo et al., (Azevedo et al., 2009) heterologously expressed AP3B1 in a kcs1Δ strain of S. cerevisiae. The protein was then extracted and incubated with [32P]PP-InsP5 in vitro (“assay input”). The [32P] (colored red) was transferred from PP-InsP5 to AP3B1 (“assay output”) which can only have occurred if AP3B1 were to have already been mono-phosphorylated by casein kinase II in vivo. Panels b, c depict two alternative explanations for the outcome of separate experiments in which AP3B1 was expressed in either wild-type or vip1Δ S. cerevisiae, which respectively contain either normal or elevated levels of PP-InsP5. AP3B1 was then extracted and incubated with [32P]PP-InsP5 in vitro (“assay input”). Little or no [32P] was transferred from [32P]PP-InsP5 to AP3B1 (“assay output”). There are two explanations for that result. Either (panel b) the AP3B1 was already transphosphorylated (colored black) in vivo, as argued by Azevedo et al., (Azevedo et al., 2009), or (panel c) as we alternately propose, the AP3B1 may not have been monophosphorylated by casein kinase II in vivo. The data do not distinguish between these two possibilities.