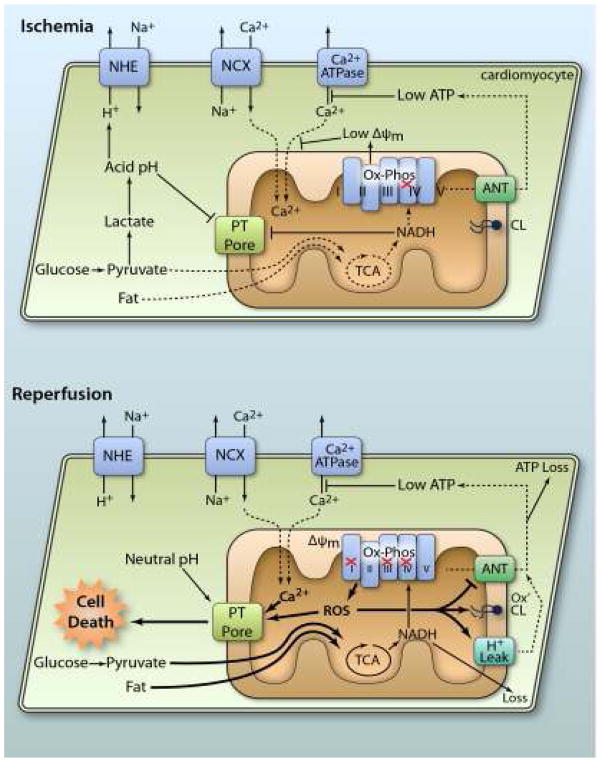
Figure 1. Mitochondrial pathologic events in IR injury.
During ischemia, lack of O2 inhibits Ox-Phos, diverting the glycolytic end product pyruvate to lactate, resulting in cellular acidification. Disposal of acid via the Na+/H+ exchanger (NHE) brings Na+ into the cytosol. This Na+ is exported via the Na+/Ca2+ exchanger (NCX) causing cytosolic Ca2+ overload, which is exacerbated by low ATP disabling Ca2+ export by ATPases. Inhibited Ox-Phos is the primary cause of low ATP. Mitochondrial Ca2+ uptake is minimal due to low ΔΨm, and both acidic pH and accumulation of NADH keeps the PT pore closed. At reperfusion, rapid re-establishment of Ox-Phos and ΔΨm results in mitochondrial Ca2+ overload and ROS generation. Together with normalization of pH, this triggers PT pore opening, leading to cell death. In surviving cells, ROS activates mitochondrial uncoupling (H+ leak), which inhibits ATP synthesis, exacerbating the metabolic crisis. Oxidation of cardiolipin (CL) also occurs, along with nucleotide loss (both NADH from mitochondria and ATP from the cell). ROS damage to ANT contributes to low ATP levels. For full explanation see text.(Illustration Credit: Ben Smith).