Abstract
Herein is reported efficient erythropoietic differentiation of a human embryonic stem cell (ESC) line derived from a preimplantation genetic diagnosis (PGD)-screened embryo that harbours the homozygous sickle cell disease (SCD) haemoglobinopathy mutation. This human ESC line possesses typical pluripotency characteristics and forms multilineage teratomas in vivo. SCD-human ESC efficiently differentiated to the haematopoietic lineage under serum-free and stromal co-culture conditions and gave rise to robust primitive and definitive erythrocytes. Expression of embryonic, fetal and adult sickle globin genes in SCD PGD-derived human ESC-derived erythrocytes was confirmed by quantitative real-time PCR, intracytoplasmic fluorescence-activated cell sorting and insitu immunostaining of PGD-derived human ESC teratoma sections. These data introduce important methodologies and paradigms for using patient-specific human ESC to generate normal and haemoglobinopathic erythroid progenitors for biomedical research.
Keywords: haematopoiesis, human embryonic stem cells, preimplantation genetic diagnosis, red blood cells, sickle cell anaemia
Introduction
Preimplantation genetic diagnosis (PGD) is routinely used in assisted reproduction to screen inherited genetic disorders in gametes or cleavage-stage embryos during IVF cycles to avoid births with severe genetic disorders. More than 100 testable genetic conditions, including severe haematological disorders such as beta-thalassaemia, Fanconi anaemia and sickle cell disease (SCD), can be PCR-screened using the polar body of an unfertilized oocyte or from a microbiopsied blastomere. The derivation of human embryonic stem cell (ESC) lines from preimplantation embryos with genetic disorders has been extensively reported, and these PGD-derived human ESC are untested, yet potentially valuable, tools for investigating cellular and molecular events of human embryogenesis in diseased states (Ben-Yosef et al., 2008; Eiges et al., 2007; Kuliev et al., 2001; Strelchenko et al., 2004; Verlinsky et al., 2005, 2006).
A more recent approach for creating human pluripotent stem cells (PSC) that harbour genetic disorders is via the generation of induced PSC using defined transgenic pluripotency factors. For example, a great deal of interest has recently been invested in modelling the developmental pathology of haematological disorders using disease-affected induced PSC and in treating haematological disorders with genetically corrected haematopoietic stem cells that are derived from autologous induced PSC (Hanna et al., 2007; Park et al., 2008a). Sickle cell haemoglobinopathy, a classic inherited monogenic disorder resulting from the substitution of glutamate to valine at position 6 of the beta-haemoglobin chain, is an important candidate for such stem cell-based therapies. A proof of principle for induced PSC-based cellular/genetic therapy was recently demonstrated in a murine model of sickle cell anaemia (Hanna et al., 2007). However, human PSC lines generated by nascent induced PSC technology may face several caveats in their differentiation capacity that may limit their use in disease modelling, such as incomplete reprogramming and viral integration effects. Thus, disease-affected, bona-fide human ESC derived from IVF-derived PGD-selected preimplantation embryos can serve as gold standards for preclinical validation of induced PSC-based therapies.
This study significantly advances several of these concepts by reporting the characterization and haematopoietic differentiation of a novel PGD-derived human ESC line harbouring the homozygous mutation for SCD haemoglobinopathy. More importantly, these data demonstrate the utility and feasibility of using patient-specific human ESC for generating erythroid progenitors for haematological disease modelling and therapeutics.
Materials and methods
Derivation, culture, characterization and genotyping of a pluripotent PGD-derived human ESC line affected with the homozygous SCD mutation
Human ESC line SC233 (NIH human ESC registry; RG-233) was established by Reproductive Genetics Institute (Chicago, IL, USA) via original techniques from donated morula-stage embryos (Ley et al., 1983; Verlinsky et al., 2006). Patients undergoing PCR-based PGD selection to avoid the homozygous SCD mutation gave institutionally approved informed consent for the IVF process, the PGD selection process, as well as for the derivation of human ESC from surplus, disease-affected embryos. All human ESC lines used in these studies were evaluated and approved by the JHUSM Embryonic Stem Cell Research Oversight (ESCRO) Committee to assure derivation a informed consent guidelines conformed with those recommended by the National Academy of Sciences for research involving human ESC. Line SC233, as well as control H1 (WA01) and H9 (WA09) lines obtained from WiCell, were cultured in standard conditions. Line SC233 was obtained and expanded at low passage (about p6).
Karyotyping was performed using high resolution G-banding (JHUSM Cytogenetics Core). In the study laboratory, line SC233 displays classic human ESC morphology in typical culture conditions and has a high level of expression for pluripotency markers (e.g., alkaline phosphatase, SSEA3, SSEA4, TRA-1–60, TRA-1–81 and OCT4 (ES Cell Marker Kit; Chemicon). The SCD beta-6 mutation in line SC233 was detected by DdeI (New England Biolabs) restriction digest of PCR-amplified genomic DNA. The SCD AT transversion point mutation was further confirmed by direct DNA sequencing of the PCR-amplified beta-globin locus. Genomic DNA from SC233 and control H1 human ESC was isolated using DNeasy reagents (Qiagen). Diluted genomic DNA was PCR-amplified using primers specific to exon I of the beta-globin locus with the following primers: forward HBB: 5′-AGC CAG TGC CAG AAG AGC-3′; reverse HBB: 5′-AGG GGA AAG AAA ACA TCA AGG GTC-3′. A specific 688-bp PCR product was purified using Qiagen spin columns, digested with DdeI and analysed on 4% agarose gels, or subcloned (TOPO XL/TA Cloning kit; Invitrogen), and subsequently sequenced directly.
Teratoma formation and immunostains
Teratomas were prepared in immunodeficient mice, as previously described (Park et al., 2008b). Immunohistochemical staining using anti-human beta-globin (Santa Cruz), anti-human CD34 and murine Ter119 (Becton Dickinson) antibodies was performed using standard protocols.
Erythroid differentiation of human ESC
Human embryoid bodies (EB) from normal (H1, WA01; H9, WA09) and SC233 ESC were differentiated as described before (Zambidis et al., 2005, 2008) but with minor modifications. ESC cultures with minimal differentiation were grown to about 60–80% confluence on gelatinised plates and irradiated primary mouse embryo fibroblasts. Twenty-four hours prior to EB formation, serum-free human ESC medium was switched to an adaptation medium (Zambidis et al., 2005, 2008) consisting of serum-free expansion medium (SFEM; StemCell Technologies) supplemented 15% fetal bovine serum (FBS; StemCell Technologies), 50 mg/ml ascorbic acid (Sigma), 1% enhancement media supplement EXCYTE (Millipore), 0.5% insulin/transferrin/selenium-X supplement (Invitrogen), 3.5% PFHM-II (Invitrogen) and 100 U/100 g penicillin-streptomycin (Invitrogen). ESC were harvested 24 h later with 1 mg/ml collagenase IV (Invitrogen) and cultured for an additional 2–3 days in 6-well ultra low-attachment plates (Corning) for EB formation. One well of ESC was transferred to 1-well ultra low-attachment plates for EB formation in 2.5–3 ml/well of methylcellulose medium (SF H4236; StemCell Technologies) supplemented with 15% FBS (StemCell Technologies), 50 μg/ml ascorbic acid, 0.5% enhancement media supplement EX-CYTE, 3.5% PFHM-II and 5–10 mmol/l Rock inhibitor (Y-27632; Calbiochem). Formed EB were collected 2–3 days later, washed with phosphate-buffered saline and replated into ultra low-attachment 6-well plates (1 well of human EB to 1–2 wells) in SFEM which consisted of all the ingredients described above for adaptation medium except FBS, supplemented with BVF2H: recombinant human bone morphogenetic protein 4 (50 ng/ml), vascular endothelial growth factor A165 (50 ng/ml), fibroblast growth factor 2 (50 ng/ml) (Invitrogen) and 5 g/ml heparan sulphate (Sigma). After 6 days, large formed EB were briefly treated with Accutase (Sigma) to form smaller disaggregated EB clumps and recultured for an additional 4 days in the same culture medium that included BVF2H, but also thrombopoietin (50 ng/ml), interleukin 6 (50 ng/ml) and erythropoietin (5 U/ml) (Invitrogen). All growth factors were purchased from R and D Systems. At day-10 EB differentiation, cultures were treated again with Accutase to make single cell suspensions, which were passed through 70 mm strainers for clonogenic colony-forming cell (CFC) assays or used directly without straining for long-term erythroid expansions, for further differentiation on human mesenchymal cell (MSC) or OP9 stromal layers.
Haematopoietic differentiation of human EB cells on OP9 or human MSC stromal layers
For OP9 human EB differentiation, fluorescence-activated cell sorting (FACS)-purified day-10 BB9+ (ACE/CD143) cells from H9 or SC233 EB, prepared as described above, were co-cultured on irradiated (2000 rad) OP9 (ATCC) mouse stromal layers and cultured in adaptation medium for 3–4 weeks with biweekly medium changes. Medium was supplemented with recombinant human growth factors (R and D Systems) including EPO (5 U/ml), FLT3L (20 ng/ml), TPO (20 ng/ml), SCF (20 ng/ml), IL-3 (20 ng/ml), IL-6 (20 ng/ml), G-CSF (20 ng/ml) and GM-CSF (20 ng/ml). Differentiated cells were used for flow cytometry analysis. OP9 cells were routinely maintained and expanded in 20% FBS (BenchMark, Gemini Bio-Products) in Alpha-MEM (Invitrogen) containing l-glutamine and 100 U/100 μg penicillin-streptomycin. For human MSC differentiation, Accutase-treated day-10 EB cells, prepared as described above, were cultured in serum-free liquid differentiation medium with these same growth factors for 1 week. These day-17 EB/human MSC cultures were evaluated for erythroid CFC potential in H4436 methylcellulose medium (StemCell Technologies) supplemented with 0.5% EX-CYTE.
Clonogenic assays, cytospin haematological stains, flow cytometry analysis and FACS purification of human EB-derived haemangioblasts
Wright–Giemsa stains of cytospun cells and CFC assays of single-cell suspensions of day-10 and day-17 human EB cells were performed in serum-free H4436 methylcellulose medium containing recombinant human growth factors were performed as described before (Zambidis et al., 2005, 2008). Surface markers BB9-APC (anti-ACE/CD143), CD36PE, CD71-CyPE, glycophorin A (CD235A)-PE and CD45-PE (BD Biosciences) were evaluated by flow cytometry on enzymically dissociated single EB cells or erythroid cells at different time points, as described (Zambidis et al., 2008). CD34+ cord blood cell-derived erythroid cells were used as a control. Fetal haemoglobin, adult beta-haemoglobin and embryonic epsilon-haemoglobin chain expressions were quantitatively evaluated by intracellular flow cytometry staining using fluorochrome-conjugated monoclonal antibodies, as previously described (Zambidis et al., 2005, 2008). The fluorochrome-conjugated monoclonal antibodies used were used as previously described (Zambidis et al., 2008) and included gamma-chain-specific anti-fetal haemoglobin-PE (Caltag), anti-embryonic epsilon-chain-FITC (clone 0900–50; Cortex Biochem) and murine anti-beta-globin chain (Santa Cruz) plus secondary PE-conjugated anti-mouse antibody (Southern Biotech).
For FACS purification of CD143+ human EB-derived haemangioblast populations (Zambidis et al., 2008), differentiated day-10 EB were enzymically disaggregated into single-cell suspension with Accutase, passaged through 40 μm strainers and immunostained on ice in serum-free medium with BB9-APC monoclonal antibody (BD Biosciences). Single, purified BB9+ EB cells were incubated in 10 μmol/l Rock inhibitor during the period of FACS collection. Post-sorted cells were additionally cultured on OP9/human MSC feeder layers, as described below, and analysed by flow cytometry for expression of haematopoietic surface markers at indicated time points.
Quantitative real-time PCR
Relative quantitative real-time PCR (qRT-PCR) analysis of haemoglobin genes was performed as previously described (Zambidis et al., 2005) using the 2–ΔΔT method of primitive erythroid colonies derived from day-10 EB cells, definitive erythroid colonies derived from day-17 MSC/EB cultures (described below), BFU-e derived from CD34+ cord blood cells and peripheral blood from normal donors or patients with sickle cell disease. To specifically detect mutant sickle globin mRNA, qRT-PCR products of the beta-globin locus were amplified, as described above, and purified with Qiagen spin columns (QIAquick Gel Extraction Kit), digested with DdeI enzyme (Fermentas; New England Biolabs) and analysed on 2% agarose gels.
Results
A PGD-derived human ESC line harbouring SCD mutations allows the direct study of the earliest phases of haemoglobinopathic erythropoiesis.
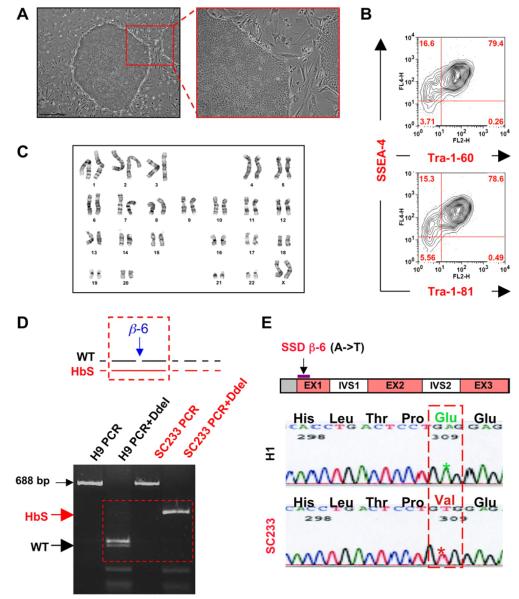
A repository of PGD-derived human ESC lines affected with various genetic disorders was previously described (Verlinsky et al., 2006). The current study evaluated a PGD-derived human ESC line (SC233) obtained from this repository, which was previously reported to harbour the homozygous SCD mutation using multiplex PCR genotyping analysis (Kuliev et al., 2001). Line SC233 was continuously maintained for at least 45 passages without spontaneous differentiation or loss of pluripotency. In the study laboratory, this line displays classic human ESC morphology in standard culture conditions, maintains a normal 46,XX female karyotype and robustly expresses typical pluripotency markers (Figure 1A–C). The current study validated homozygous SCD point mutations in line SC233 via both DdeI analysis of PCR-amplified genomic DNA of the beta-globin locus (Figure 1D) as well as by direct sequencing of the PCR-amplified genomic beta-globin locus (Figure 1E).
Figure 1.
Characterization of pluripotent preimplantation genetic diagnosis-derived human embryonic stem cell (ESC) line SC233 harbouring homozygous sickle cell disease (SCD) mutations. (A) SC233 demonstrates typical human ESC morphology under standard culture conditions (bar = 500 lm) and (B) displays representative pluripotency surface marker staining (e.g., SSEA4, Tra-1–60, TRA-1–81 surface) by fluorescence-activated cell sorting analysis. (C) High resolution G-banding of SC233 revealed a normal, female XX karyotype. (D) SC233 tested positive for homozygous SCD mutations as assessed by DdeI restriction enzyme digestion of PCR-amplified genomic DNA of the beta-globin locus. (E) Sequencing analysis of subcloned PCR products (687 bp) amplified from the genomic beta-globin locus revealed homozygous sickle cell haemoglobin (HbS) mutations. Genomic DNA from either normal human ESC line H1 or SC233 were used as PCR templates. Ten out of 10 SC233 beta-globin locus subclones contained the classic A → T point mutation, which converts polar glutamate into a hydrophic valine at position β-6. EX = executer; H1 = WA01 control; HbS = sickle cell haemoglobin; IVS = intervening sequence; WT = wild type.
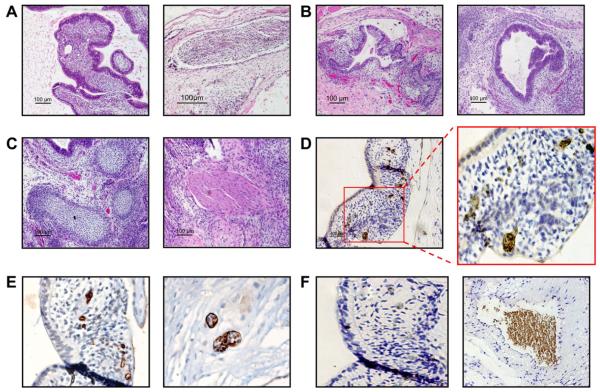
Line SC233 forms multilineage teratomas containing elements of all three germ layers following injection into immunodeficient mice (Figure 2A–C). SC233 teratomas contained areas of haematoendothelial differentiation, including vascularized mesoderm containing CD34+ structures reminiscent of haemangioblastic clusters observed during haematopoietic genesis in the human embryo (Tavian et al., 1999). Moreover, these CD34+ haemangioblastic structures formed blood island-like foci that contained adult haemoglobin-expressing erythroid cells (Figure 2D–F).
Figure 2.
Detection of ectodermal, endodermal, mesodermal and primitive haematopoieic differentiation in xenografted pluripotent preimplantation genetic diagnosis-derived human embryonic stem cell line SC233 teratomas. Injection of SC233 into highly immunodeficient NOG mice gave rise to teratomas containing differentiated derivatives of all three embryonic germ layers. (A–C) Representative haematoxylin and eosin stains from mature, cystic SC233 teratoma sections demonstrate pluripotent differentiation to (A) ectoderm (neural differentiation), (B) endoderm (left panel, gut; right panel, respiratory epithelial), and (C) mesoderm (left panel, cartilage; right panel, muscle) (bars = 100 μm). (D) Rare areas of haematopoietic differentiation in mesodermal areas were detected in SC233 teratoma sections by in-situ immunostaining of adult haemoglobin-expressing erythrocytes (bar = 400 μm; right panel magnification 400×). (E) Associated CD34+ haemangioblastic structures in teratoma sections, many of which contained differentiating clusters of erythroid cells (left panel magnification 200×; right panel 400×). (F) Haematopoietic cells were confirmed to be human teratoma-derived cells (and not intermixed host murine erythroid cells) via virtue of negative staining for murine erythroid cell-specific Ter119 expression (left panel, magnification 200×). Strongly positive Ter119 staining of intermixed murine cells is shown in the right panel in an adjacent area of the same slide (magnification 200×).
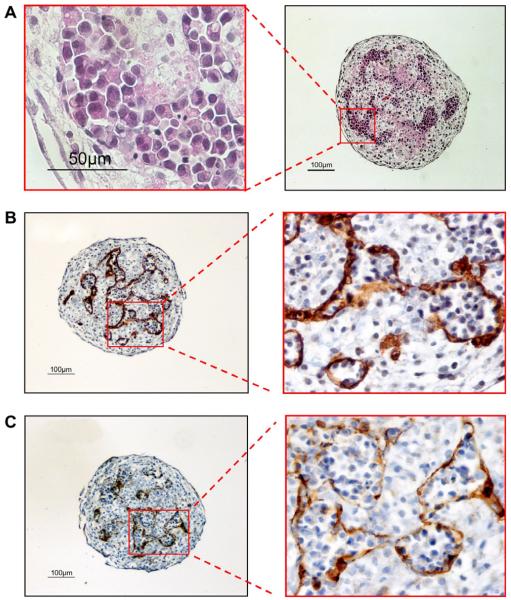
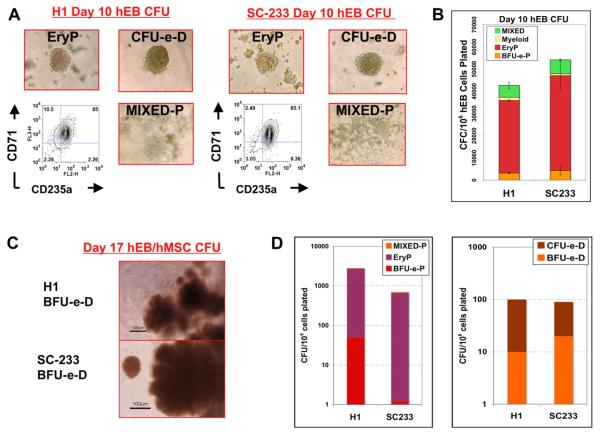
To evaluate the erythropoietic potential of the PGD-derived human ESC line SC233, haematopoietic differentiation was first performed via formation of EB in serum-free medium supplemented with haematopoietic growth factors, as previously described (Zambidis et al., 2008). Under these differentiation conditions, SC233 ESC formed typical cystic EB and, after several weeks of differentiation, EB developed foci of proliferating nucleated erythroblasts that were contained within CD34+CD31+-expressing endothelium-lined sacs (Figure 3). Remarkably, these well-organized structures were morphologically similar to the yolk sac blood islands typically observed in human embryos at about 19 days of development (Tavian et al., 1999). Single, disaggregated cells from these EB, or from normal H1 (WA01) control ESC, were assayed for haematopoietic progenitors in serum-free methylcellulose CFC cultures. Day-10 SC233 EB gave rise to primitive progenitors as robustly as control H1 EB and formed the full range of primitive yolk sac-like colony-forming units (CFU) previously described for normal human ESC (mixed erythro-myeloid, myeloid (macrophage) and primitive erythroid CFU (EryP, BFU-e-P; Figure 4A–B) (Zambidis et al., 2005, 2008).
Figure 3.
Yolk sac-like blood island development in pluripotent preimplantation genetic diagnosis-derived human embryonic stem cell line SC233 embryoid bodies. Robust yolk sac-like primitive erythropoietic differentiation develops within SC233 embryoid bodies differentiated for 19–21 days. (A) Haematoxylin and eosin staining of sectioned whole SC233 embryoid bodies revealed yolk sac-like blood islands contained within endothelial-lined sacs that immunostained with (B) CD34 and (C) CD31 antibodies. (A) Left panel bar = 50 lm, right panel bar = 100 μm; (B) left panel bar = 100 μm, right panel magnification, inset: 400 × magnification; (C) left panel bar = 100 μm, right panel magnification, inset: 400 × magnification.
Figure 4.
Primitive and definitive erythropoietic differentiation from pluripotent preimplantation genetic diagnosis-derived human embryonic stem cell line SC233 embryoid body cells. Single-cell suspensions from day-10 SC233 or control H1 EB cells produced comparable (A) morphologies and frequencies (B) of yolk sac-like primitive haematopoietic progenitors in methylcellulose colony assays. Colony-forming units per 106 single human EB cells plated were scored by morphology, as described (Zambidis et al., 2005, 2008). (C) Erythroid colony-forming units with a fetal liver-like definitive morphology (CFU-e-D, BFU-e-D) were generated at robust frequencies (D) from H1 and SC233 human EB cells following further differentiation on human mesenchymal cell stromal layers. BFU-e = burst-forming unit erythroid; CFC = colony-forming cell; CFU = colony-forming unit; EryP = primitive erythroid CFU; H1 = WA01 control; hEB = human embryoid body; hMSC = human mesenchymal cell; MIXED = macrophage (myeloid).
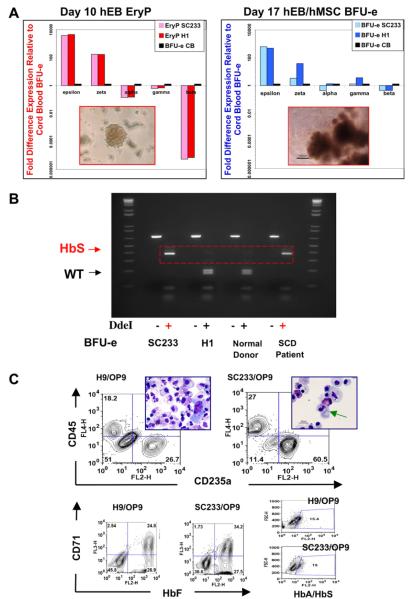
To generate adult beta-haemoglobin-expressing erythroid progenitors, disaggregated, single day-10 SC233 or H1 EB cells were co-cultured on human MSC feeder layers in the presence of haematopoietic growth factors. Differentiated EB cells were collected for CFC assays 1 week later (day-17 EB/human MSC). Erythroid CFU from SC233 or control H1 EB expanded in methylcellulose cultures in comparable frequencies and were scored as primitive or definitive by morphology and haemoglobinization patterns (Zambidis et al., 2008) (Figure 4C–D). Erythroid colonies from these primitive and definitive differentiation cultures were picked and pooled and their RNA was evaluated for haemoglobin chain expression by qRT-PCR analysis (Zambidis et al., 2005) (Figure 5A). Primitive day-10 erythroid colonies (EryP, BFU-e-P) with yolk sac-like haemoglobin patterns expressed highly abundant amounts of embryonic epsilon-haemoglobin and zeta-haemoglobin chains (about 100- to 5000-fold higher than control cord blood CFU-e). Moreover, these colonies expressed low concentrations of fetal haemoglobins (alpha and gamma chains) and undetectable quantities of adult beta-haemoglobin chains. In contrast, erythroid CFU that were scored as definitive (CFU-e-D, BFU-e-D) from day-17 EB/human MSC cultures expressed increased fetal haemoglobins (in amounts comparable to cord blood CFU-e) and considerably increased concentrations of adult beta-globin chains (albeit lower than concentrations in cord blood CFU-e). However, these fetal and adult haemoglobin-expressing erythroid cells also continued to express abundant amounts of embryonic epsilon and zeta globins. Thus, despite adult beta-globin expression, these definitive-type erythroid colonies remained at a development stage akin to the late yolk sac or early fetal liver phase of erythropoiesis. Finally, to confirm that there was expression of mutant sickle beta-haemoglobin in these day-17 definitive SC233 erythrocytes, the mRNA from the beta-globin-expressing definitive-type erythroid cells was assayed with qRT-PCR. PCR products of amplified beta-globin loci were digested with DdeI enzyme to confirm that pooled CFU-e-D colonies derived from SC233 EB (but not control H1 EB) expressed exclusively mutant sickle beta-globin (Figure 5B).
Figure 5.
Expression of embryonic, fetal and adult haemoglobins in differentiated primitive and definitive pluripotent preimplantation genetic diagnosis-derived human embryonic stem cell line SC233 erythroid cells. (A) Comparative haemoglobin gene expression analysis of normal (H1) and mutant (SC233) erythroid cells by quantitative real-time PCR (qRT-PCR). Day-10 embryoid body primitive colonies (EryP), day-17 embryoid body/human mesenchymal cell co-culture erythroid colonies (BFU-e) and BFU-e colonies derived from control CD34+ neonatal cord blood cells were picked from methylcellulose cultures and pooled for RNA isolation and qRT-PCR. Expression of embryonic haemoglobins (epsilon, zeta), fetal haemoglobins (alpha, gamma) and adult haemoglobins (alpha, beta) was quantified using the 2 ΔT method (Zambidis et al., 2005, 2008). Results are shown as fold differences relative to haemoglobin expressions in control cord blood BFU-e (normalized to a value of 1). Left inset = EryP; right inset = BFU-e. (B) Expression of sickle cell haemoglobin in SC233 erythroid cells. RNA derived from SC233 BFU-e, H1 BFU-e, normal donor peripheral blood and SCD patient peripheral blood was isolated and analysed by RT-PCR. A 687 bp PCR product was amplified using beta-globin locus primers. PCR products digested with DdeI and fractionated on agarose gels with or without enzyme digestion (–/+). (C) Haematopoietic differentiation of control H1 or SC233 ACE/CD143+ day-10 embryoid body cells on OP9 stromal layer cells produced morphologically comparably definitive-type erythro-myeloid differentiation several weeks later. The amounts of CD45+ myeloid and megakaryocytic and CD235a+ definitive-type erythroid cells are comparably robust from both H9 and SC233 embryoid body cells. Both embryoid body cells generated definitive-type erythrocytes on OP9 stromal cells (some with evidence of enucleation; green arrow) that had increased liver-like fetal haemoglobin expression, but minor amounts of adult haemoglobin expression. BFU-e = burst-forming unit erythroid; EryP = primitive erythroid CFU; H1 = WA01 control; HbA = adult haemoglobin; HbF = fetal haemoglobin; HbS = sickle cell haemoglobin; hEB = human embryoid body; hMSC – human mesenchymal cell; SCD = sickle cell disease; WT = wild type.
To further confirm that line SC233 has haematopoietic differentiation capacity comparable to the standard human ESC lines used in the study laboratory, haematopoietic differentiation of SC233 and control H9 (WA09) human ESC was induced in liquid OP9 stromal layers supplemented with haematopoietic growth factors (Zambidis et al., 2008). Day-10 CD143+ EB cells (which contain haemangioblast populations that give rise to yolk sac-like primitive and definitive erythro-myelopoiesis; Zambidis et al., 2008) from SC233 ESC differentiated with similar efficiency to control H9 CD143+ EB cells on OP9 stromal layers. Cultures from control H9 and SC233 EB cells contained similar amounts of erythrocytes, granulocytes, macrophages and megakaryocytes (Figure 5C).
Discussion
This study reports for the first time, as far as is known, the efficient erythropoietic differentiation of a novel diseased PGD-derived human ESC harbouring homozygous SCD mutations. It has also demonstrated the general utility of PGD-derived human ESC for modelling the developmental pathology of human haemoglobinopathies. The eventual mass expansion of normal or haemoglobinopathic erythroid progenitors should have broad application in various molecular, developmental, gene therapeutic, pharmacological, microbiological and disease-modelling studies. PGD-derived human ESC lines affected with other haematological disorders such as alpha- and beta-thalassaemias and Fanconi anaemia have also been derived (Ben-Yosef et al., 2008; Verlinsky et al., 2005. 2006) and they represent valuable resources for studying developmentally regulated pathology that is otherwise impossible to study in animal models or with limited human embryonic tissues. However, the great majority of currently available PGD-derived human ESC lines remain uncharacterized. In addition to several recently described patient-specific induced PSC lines that harbour haematological lesions (Park et al., 2008a), and in combination with previously reported PGD-derived human ESC (Ben-Yosef et al., 2008; Verlinsky et al., 2005, 2006), these PSC represent valuable resources for modelling human blood disorders.
First-generation human induced PSC lines have been derived by integrating retroviral transgenes (e.g., SOX2, OCT4, KLF4, C-MYC, LIN-28 and NANOG) (Dimos et al., 2008; Nakagawa et al., 2008; Park et al., 2008a,b; Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Yu et al., 2007). Although retroviral proto-oncogenic transgenes are partially silenced in undifferentiated induced PSC, they still retain potential for insertional mutagenesis or low-level ectopic expression during differentiation. Additionally, ectopic expression of pluripotency factors during differentiation has the potential to produce unexpected effects that might be attributed incorrectly to the diseased phenotype. Moreover, it remains to be demonstrated that individual human induced PSC lines, including those that form multilineage teratomas and express classic pluripotency markers, have achieved full pluripotent reprogramming and are capable of normal haematopoietic differentiation. For example, recent reports demonstrated that, in comparison to blastocyst-derived human ESC, both human and murine induced PSC clones possess variable efficiencies in their ability to differentiate to the haematopoietic lineage, even after being derived from the same donor fibroblasts or with the same pluripotency transgenes (Choi et al., 2009; Hanna et al., 2007). The technical variables currently associated with these first-generation induced PSC are completely bypassed by the use of PGD-derived human ESC, which are bona-fide diseased human ESC derived from preimplantation embryos. However, induced PSC lines are ultimately expected to be a more powerful resource since they can provide a much wider bank of diseased PSC than the limited repertoire of PGD-derived human ESC lines currently available. Since improved nonviral methodologies for generating induced PSC are currently under investigation (Okita et al., 2008; Stadtfeld et al., 2008), second-generation induced PSC will likely be utilized synergistically with PGD-derived human ESC lines for validating their accuracy in modelling human developmental pathology.
Despite recent advances for generating patient-derived PSC for haematological disease modelling, these studies also underscore that major challenges still remain for elucidating the pathways of differentiation toward erythroid and other haematopoietic lineages. For example, methods for the generation of long-term engrafting haematopoietic stem cells, or adult-type lymphoid and erythroid lineages from human ESC or induced PSC, have yet to be elucidated. The nature of this problem may lie in the fact that human ESC differentiation produces primitive haematopoiesis similar to that found in the human yolk sac, which has poor long-term engraftment ability in adult recipients (Zambidis et al., 2005, 2008). The current study, similarly to previous studies with normal human ESC (Lu et al., 2008; Olivier et al., 2006; Qiu et al., 2008), demonstrated that fetal-type diseased erythrocytes originated from human ESC-derived embryonic haemangioblasts (Lu et al., 2008; Olivier et al., 2006; Qiu et al., 2008; Zambidis et al., 2008). A sophisticated developmental biological approach is ultimately necessary for differentiating human ESC and induced PSC into clinically relevant adult-type haematopoietic lineages that normally develop in adult bone marrows. For instance, the efficient large-scale production of adult-type normal and diseased red blood cells from human ESC and induced PSC could provide a valuable standardized resource for experimental haematological studies if optimized for mass production, as has been done in murine ESC systems. However, this goal may require further investigation into human ESC differentiation methods for mass-producing erythrocytes that express adult beta-globin (Carotta et al., 2004; Chang et al., 2006; Ma et al., 2008; HiroyaMa et al., 2008).
The current study devised novel differentiation protocols for generating immature fetal-type human erythroid progenitors. These erythrocytes expressed low concentrations of adult beta-globin (<20%) but high concentrations of embryonic/fetal haemoglobins and are thus currently limited in their ability to model beta-haemoglobinopathies such as SCD. Efficient protocols for large-scale expansion of mature adult-type, beta-globin-expressing red blood cells from human ESC have not yet been reported in the literature and remain to be developed (as they have been for cord blood CD34+ cells (Giarratana et al., 2005; Miharada et al., 2006; Neildez-Nguyen et al., 2002)). Such methodologies will likely depend on recapitulating the in-vivo microenvironment found in the fetal liver and adult bone marrow (Ma et al., 2008) and will be necessary before diseased PGD-derived human ESC and induced PSC can reach their potential for modelling beta-haemoglobinopathies. Moreover, the mass production of haemoglobinopathic fetal erythroblasts will likely be an important experimental resource for molecular, gene-therapy and drug-testing studies in haematological research. Ultimately, genetically abnormal human PSC lines derived via both PGD-screening and induced PSC generation, if used in a complementary fashion, can synergistically advance human disease modelling or preclinical testing of induced PSC-based therapies. For example, PGD-derived human ESC affected with haemoglobinopathies can serve as gold standards for haematopoietic differentiation and genetic correction strategies of newly generated patient-specific induced PSC. Ultimately, PGD-derived human ESC and induced PSC lines will serve as valuable resources for the mass generation of haemoglobinopathic erythroid progenitors for disease modelling and drug testing.
Acknowledgements
This research was supported entirely from grants provided by the Maryland Stem Cell Research Fund (E.T.Z. and M.V.P.) and the Institute for Cell Engineering, JHUSM (E.T.Z.). The authors thank Rajni Sharma for expert immunostaining of teratoma tissue sections and Xuan Yuan, Paul Burridge, Tea Soon Park and Michal Levine for technical assistance in these studies.
Biography

Marina V Pryzhkova completed her PhD in Molecular Biology in 2004 at the Institute of Gene Biology, RAS, Moscow, Russia, She continued to work in the stem cell field at Lifeline Cell Technology, Walkersville, MD, USA. She is currently a postdoctoral fellow at the Institute for Cell Engineering at the Johns Hopkins School of Medicine, MD, USA. Her current research includes the development of methods for long-term expansion and genetic modification of erythroid progenitors from human embryonic stem cells and induced pluripotent stem cells.
Footnotes
Declaration:
The authors report no financial or commercial conflicts of interest.
References
- Ben-Yosef D, Malcov M, Eiges R. PGD-derived human embryonic stem cell lines as a powerful tool for the study of human genetic disorders. Mol. Cell. Endocrinol. 2008;282:153–158. doi: 10.1016/j.mce.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Carotta S, Pilat S, Mairhofer A, et al. Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood. 2004;104:1873–1880. doi: 10.1182/blood-2004-02-0570. [DOI] [PubMed] [Google Scholar]
- Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science (New York, N.Y.) 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Eiges R, Urbach A, Malcov M, et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat. Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science (New York, N.Y.) 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hiroyama T, Miharada K, Sudo K, et al. Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS ONE. 2008;3:e1544. doi: 10.1371/journal.pone.0001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliev A, Rechitsky S, Verlinsky O, et al. Preembryonic diagnosis for sickle cell disease. Mol. Cell. Endocrinol. 2001;183(Suppl. 1):S19–S22. doi: 10.1016/s0303-7207(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Ley TJ, DeSimone J, Noguchi CT, et al. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion ofdense cells in patients with sickle cell anemia. Blood. 1983;62:370–380. [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc. Natl Acad. Sci. USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miharada K, Hiroyama T, Sudo K, et al. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat. Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Neildez-Nguyen TM, Wajcman H, Marden MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat. Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science (New York, N.Y.) 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Olivier EN, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp. Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008a;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008b;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science (New York, N.Y.) 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelchenko N, Verlinsky O, Kukharenko V, et al. Morula-derived human embryonic stem cells. Reprod. Biomed. Online. 2004;9:623–629. doi: 10.1016/s1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tavian M, Hallais MF, Peault B. Emergence of intraem-bryonic hematopoietic precursors in the pre-liver human embryo. Development (Cambridge, England) 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Strelchenko N, Kukharenko V, et al. Human embryonic stem cell lines with genetic disorders. Reprod. Biomed. Online. 2005;10:105–110. doi: 10.1016/s1472-6483(10)60810-3. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Strelchenko N, Kukharenko V, et al. Repository of human embryonic stem cell lines and development of individual specific lines using stembrid technology. Reprod. Biomed. Online. 2006;13:547–550. doi: 10.1016/s1472-6483(10)60643-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y.) 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]