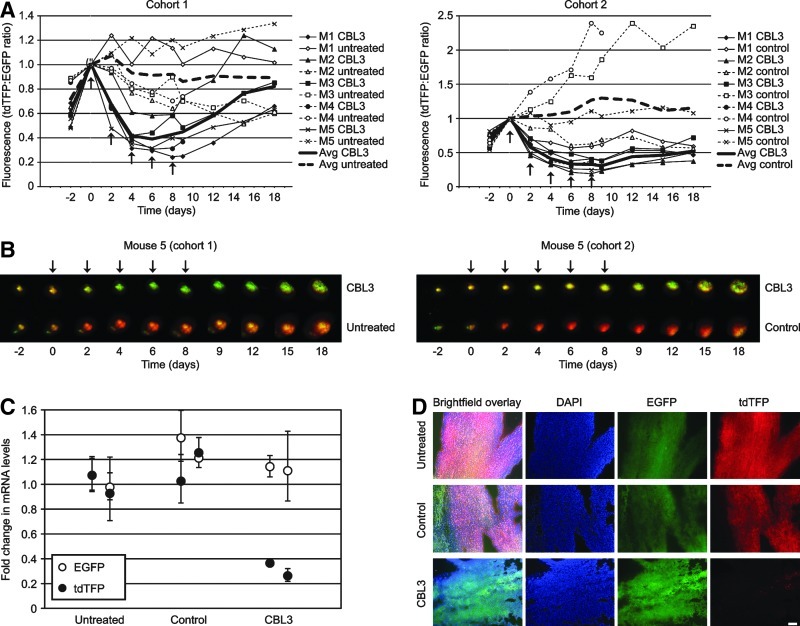
FIG. 2.
Specific siRNA (CBL3) inhibits reporter expression in a dual fluorescent reporter melanoma xenograft model. (A) Human A375 melanoma cells expressing EGFP and tdTFP were injected (1×106 cells per injection) into both the right and left flanks of 10 immunocompromised mice, which were divided into two cohorts of 5 mice each. The resulting tumors were intravitally imaged as indicated (see Materials and Methods) to measure EGFP and tdTFP expression levels. The tumors were treated with 0.5 mg/mL siRNA/IVF2.0 complex, containing either nonspecific control or CBL3 siRNA, every 2 days for a total of 5 treatments (indicated by arrows). tdTFP fluorescence was normalized to EGFP fluorescence to account for differences in tumor size. All data were normalized to the “day 0” time point. (B) Overlaid tdTFP and EGFP fluorescence images from each time point are shown for mouse five (M5) from each cohort. These images reveal uniform loss of signal over the surface of the tumor, suggesting knockdown throughout the tissue and not along needle tracks alone. (C) A representative mouse from each cohort (mouse four, M4) was sacrificed on day 9 and duplicate samples were taken from each excised tumor. Total RNA from each sample was isolated and reverse transcribed. Subsequently, Luc2/tdTFP and EGFP cDNA levels (fold change relative to the untreated control) were determined relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by quantitative polymerase chain reaction (qPCR) using the delta delta cycle threshold (ΔΔCT) method. (D) An additional sample of each tumor from mouse 4 (M4) from each cohort was sectioned for analysis by fluorescence microscopy. Representative images are shown. All fluorescent images were collected using the same exposure time to allow effective comparison. Scale bar=100 μm.