ABSTRACT
Spermatogenic cell differentiation involves changes in the concentration of cytoplasmic Ca2+ ([Ca2+]i); however, very few studies exist on [Ca2+]i dynamics in these cells. Other tissues display Ca2+ oscillations involving multicellular functional arrangements. These phenomena have been studied in acute slice preparations that preserve tissue architecture and intercellular communications. Here we report the implementation of intracellular Ca2+ imaging in a sliced seminiferous tubule (SST) preparation to visualize [Ca2+]i changes of living germ cells in situ within the SST preparation. Ca2+ imaging revealed that a subpopulation of male germ cells display spontaneous [Ca2+]i fluctuations resulting from Ca2+ entry possibly throughout CaV3 channels. These [Ca2+]i fluctuation patterns are also present in single acutely dissociated germ cells, but they differ from those recorded from germ cells in the SST preparation. Often, spontaneous Ca2+ fluctuations of spermatogenic cells in the SST occur synchronously, so that clusters of cells can display Ca2+ oscillations for at least 10 min. Synchronous Ca2+ oscillations could be mediated by intercellular communication via gap junctions, although intercellular bridges could also be involved. We also observed an increase in [Ca2+]i after testosterone application, suggesting the presence of functional Sertoli cells in the SST. In summary, we believe that the SST preparation is suitable to explore the physiology of spermatogenic cells in their natural environment, within the seminiferous tubules, in particular Ca2+ signaling phenomena, functional cell-cell communication, and multicellular functional arrangements.
Keywords: calcium, cell coupling, intercellular communication, spermatogenesis, testis
Male germ cells display coupled spontaneous Ca2+ oscillations recorded in seminiferous tubules slices; these oscillations are due mainly to Ca2+ entry through CaV channels, and gap junctions participate in this coupling.
INTRODUCTION
Spermatogenesis is a complex process that drives the differentiation of diploid spermatogonial cells into haploid mature spermatozoa in the testis. Spermatogenesis takes place in the seminiferous tubules under strict physiological regulation that includes the interplay of paracrine hormones and intercellular communication among several cell types from the testis epithelium [1]. Inside the seminiferous tubules, Sertoli cells provide structural support and nourishment to spermatogenic cells through their secretory factors [2]. The correct interaction between Sertoli and spermatogenic cells is fundamental for the proper development of spermatogenesis. Disruption of these interactions can alter spermatogenesis and lead to infertility [3].
Ca2+ is a universal second messenger that regulates diverse physiological functions such as contraction, secretion, metabolism, proliferation, etc. [4]. Little is known about how Ca2+ regulates spermatogenesis and about the homeostatic mechanisms that control cytoplasmic Ca2+ concentration ([Ca2+]i) in cells from the seminiferous tubules. It is known that Sertoli cells display [Ca2+]i increases in response to testosterone (T) [5] and follicle-stimulating hormone [6], two critical hormones that regulate spermatogenesis. This [Ca2+]i increase mainly results from Ca2+ entry, which then regulates cell migration and proliferation [7]. Immunocytochemical and RT-PCR studies have shown that spermatogenic cells express ryanodine and inositol trisphosphate (InsP3) receptors [8–10], as well as voltage-dependent Ca2+ channels, mainly CaV3 channels [11–14]. CaV3 channels are functionally expressed in spermatogenic cells, as shown by electrophysiological recordings [11, 15]. Furthermore, fluorometric experiments in rat round spermatids have shown [Ca2+]i increases due to Ca2+ entry, as well as Ca2+ release from intracellular stores triggered by thapsigargin and ionomycin [16]. As in many cell types, [Ca2+]i changes also regulate many of the main functions of mature sperm (reviewed in [13]).
Depending on the cell type and the physiological event, [Ca2+]i increases can be transient, sustained, or oscillatory. Ca2+ oscillations are present in many endocrine tissues, such as pituitary [17], pancreas [18], and adrenal medullae [19], where they regulate basal hormone secretion [20]. In many cases, Ca2+ oscillations, whether spontaneous or stimulated, involve multicellular functional networks [21]. Recording of Ca2+ oscillations and other functional studies in such multicellular networks is only possible in intact organs or acute tissue slices. Unlike cultured or dispersed cells, these biological preparations preserve intercellular communications and the native repertoire of ionic channels, closely resembling the physiological environment of cells in situ.
The seminiferous epithelium has several types of intercellular junctions, such as tight and adherent junctions, which regulate the onset and maintenance of spermatogenesis [22, 23]. Gap junctions (GJs) allow another type of intercellular communication, in which plasma membrane channels directly interchange small molecules of <1 kDa (IP3, Ca2+, and cAMP) between connected cells [24, 25]. Connexins, structural proteins of GJs, are expressed in the seminiferous epithelium. Specifically, connexin 43 is abundantly expressed in rodent Sertoli and germ cells [26, 27]. The functionality of GJs in the seminiferous tubules has been assessed by diffusion of the fluorescent dye between pairs of Sertoli cells and between Sertoli and spermatogenic cells [26, 28]. Recently, it has been suggested that GJ couplings are involved in proliferation and apoptosis in mice testis [29]. Intercellular bridges (IBs), which result from the incomplete cytoplasm division of a group of germ cells, allow another type of cell-cell communication between syncytia of spermatogenic cells. Cells within a syncytium remain connected until they mature into fully differentiated spermatozoa [30]. The physiological role of the syncytium remains unexplained. Nonetheless, it is known that IBs participate in regulating the synchronous spermatid differentiation during spermatogenesis [31, 32].
Given the complex intercellular interactions in the seminiferous tubules, we implemented an acute slice preparation to characterize Ca2+-signaling mechanisms in spermatogenic cells, in a biological context where intra- and intercellular communications are well preserved. Here we combine the sliced seminiferous tubule (SST) preparation with fluorescence Ca2+ imaging to visualize in real-time the Ca2+ fluctuations of germ cells inside the seminiferous tubules, in both basal and stimulated conditions.
Our results show for the first time remarkable oscillatory [Ca2+]i changes in spermatogenic cells in a suitable preparation to explore their physiological behavior inside the acutely isolated seminiferous tubules.
MATERIALS AND METHODS
SST Preparation
Animal studies were performed under an institutional protocol similar to the USPHS: Guide for the Care and Use of Laboratory Animals and the Official Mexican Guide for the Care and Use of Laboratory Animals from the Secretary of Agriculture (SAGARPA NOM-062-Z00-1999). Adult Balb-c mice of 8 wk of age were used for these studies. Mice were maintained in the institute's animal facility and fed ad libitum. The animals were euthanized by decapitation under isoflurane anesthesia and testes were obtained by dissection under a stereomicroscope. The tunica albuginea of both testes was removed and the seminiferous tubules were mechanically untangled and dispersed with tweezers in a physiological solution that contained (mM) 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 12 glucose; gassed with 5% CO2, 95% O2; adjusted to pH 7.4; and embedded in 3% low-melting-point agarose (Invitrogen Catlab) dissolved in physiological solution at 25°C. The agarose containing the seminiferous tubules was hardened by immersion in ice-cold physiological solution, and the agar block was glued with cyanoacrylate onto the plate of a vibrating-blade microtome (Leica VT-1000S; Leica Microsystems CMS GmbH). Then, the slicing chamber was filled with physiological solution kept at 3°C and transverse slices of 160 μm in thickness were cut using a vibrating-blade microtome. As the seminiferous tubules are embedded in a random orientation, the tubules can be cut on sagittal, lateral, transversal, or diagonal orientations. Freshly cut SST were immediately transferred to an incubation beaker containing physiological solution at room temperature, and continuously gassed (5% CO2, 95% O2) to maintain pH near 7.4. Slices remained viable for up to 5 h after preparation.
Spermatogenic Cell Dissociation Protocol
Freshly dissociated spermatogenic cells were obtained by sequential 10-min enzymatic digestions of the testis (without tunica, with collagenase [0.5 mg/ml] and trypsin [1 mg/ml]) in the same physiological medium as above. The cell suspension was washed once with 0.5% BSA and twice with medium without BSA. Fluo-4 AM loading and Ca2+ imaging were performed as in SST preparation.
Intracellular Ca2+ Imaging of Living Testis Slices
An SST was mounted on a plexiglass recording chamber and incubated for 30 min with physiological solution containing the cell-permeable fluorescent Ca2+ indicator fluo-4AM (20 μM; Invitrogen). Then, the slice was immobilized with a nylon mesh, placed on the stage of a microscope, and continuously perfused (2 ml/min) with gassed physiological solution. Experiments were performed at room temperature. The slice was viewed with an upright microscope (Nikon Eclipse 80i) and water-immersion fluorescence Nikon objectives (10×, 20×, and 40×). Fluo-4 was excited at 488 nm with monochromatic light (Polychrome V Illumination System; TILL Photonics), and emitted fluorescence was band passed with a Nikon a B2-E/C filter set. Fluorescent images were acquired with a cooled digital CCD camera (Imago QE; TILL Photonic) under protocols written in TILL vision software 4.0. Images were acquired every second with an exposure/illumination time of 10 ms for a total of 10 min (600 images).
Image Recording and Analysis
Image sequences (movies) were obtained under basal conditions during continuous perfusion with physiological saline. Movies were processed and analyzed with macros written in Image J (Version 1.38; National Institutes of Health). Raw movies were converted to F = F(i) − F0, where F0 is the fluorescence image formed by averaging the first 5 frames of the sequence and F(i) represents each fluorescence image of the sequence. No attempts were made to calculate absolute [Ca2+]i from these data. An F(t) multicell plot from dozens of individual cells was generated with Igor Pro 5.03 macros (Wavemetrics, Inc.) written by León Islas, Ph.D. Facultad de Medicina UN-AM. Here, ordinates represent cell number (one cell per row), the time is represented in the abscissa, and F values are color coded. Numerical data were plotted with Origin 6.0 (MicroCal Software) or Igor Pro.
Analysis of the Coupling of Spontaneous Ca2+ Oscillations
For each SST movie recorded, regions of interest were drawn by hand (one region per cell) and single-cell Ca2+ dynamics calculated over 600 sec. The data were exported to R software to perform principal component analysis (PCA) with the function prcomp [33].
PCA is a mathematical procedure that uses an orthogonal transformation to convert a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables called principal components [34]. PCA transforms the data to a new coordinate system such that the greatest variance by any projection of the data comes to lie on the first principal component (PC1), the second greatest variance on the second principal component (PC2), and so on. The analysis of at least three SST preparations revealed that ≈80% of data variability is accounted by the first three principal components, PC1, PC2, and PC3 (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). To identify groups of correlation (cells showing coupled Ca2+ dynamics) we calculated a correlation coefficient (Dcorr, a “correlation distance”) between all possible paired combinations of the corresponding eigenvalues (or magnitudes) of the first three principal components. All paired combinations with a Dcor <0.15 were considered as being part of the same group of correlation (Supplemental Fig. S2). Finally, each single-cell Ca2+ dynamics was sorted according to the identified groups of correlation and plotted against its position on the SST preparation.
Drugs Applied
Niquel (Ni2+), mibefradil, T (Sigma-Aldrich), and thapsigargin (Alomone) were applied on the perfusion system during recordings, and 18α-glycyrrhetinic acid (Sigma-Aldrich) was incubated during 10 min between control and experimental condition. A 3× concentration of KCl (J.T. Baker) was applied (200 μl) into the bath using a pipette; the final concentration of KCl was 120 mM into the chamber recording. The final Ca2+ concentration present in Ringer without added Ca2+ was determined using Fura-2 and was between 0.5 and 1 μM.
RESULTS
SST Preparation
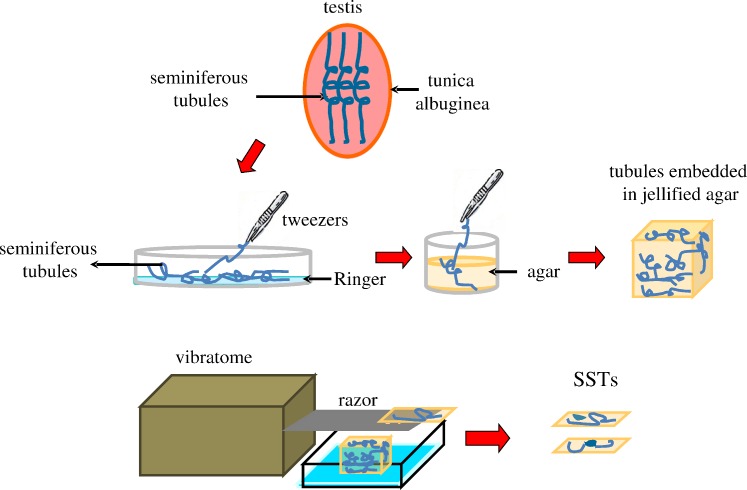
Acute tissue slices allow functional studies of several organs such as brain, cerebellum, adrenal medullae, and pituitary gland. Their use is adequate for examining functional multicellular networks and interactions between neighboring cells, a phenomenon that cannot be observed when cells are dissociated [21]. Technical approaches to obtain slices depend on the natural firmness of each particular tissue, as tissues have to be mounted on the plate of a vibratome and/or embedded in agar, as is the case for very soft tissues, to obtain mechanical stability during cutting. In this work we implemented for the first time the SST preparation from mouse testis. Figure 1 illustrates the procedure used (see Materials and Methods).
FIG. 1.
Seminiferous tubule slice technique. Cartoon showing the methodological procedures followed for obtaining SSTs. Once the tunica albuginea is removed from mice testis, seminiferous tubules are mechanically dispersed with tweezers in a Petri dish containing Ringer solution and the dispersed tubules are embedded in agar. The agar cube is mounted on the plate of a vibratome where 160-μm-thick slices are obtained.
Seminiferous Tubules Slices Partially Retain the Tissue Structure
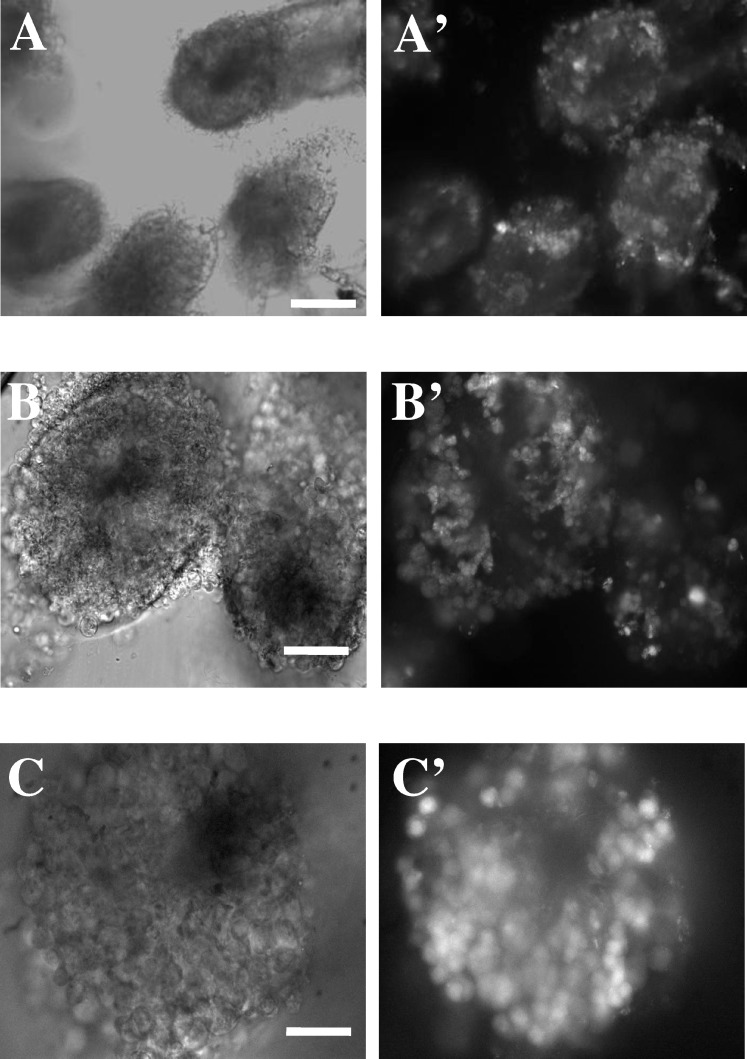
Seminiferous tubules were cut in several orientations, but for this study we used SSTs cut in a transversal orientation, that is, where the lumen of the seminiferous tubules can be identified. Histological transverse sections from seminiferous tubules (kindly provided by Diana Millán, Department of Cognitive Neuroscience, Institute of Cellular Physiology, UNAM, Mexico) established an anatomical reference to understand the distribution of germ cells in the freshly cut tubules. Accordingly, more differentiated germ cells are closer to the lumen and less differentiated cells are found towards the basal lamina of the seminiferous tubules. However, as shown in Figure 2B′, in the transversal SST preparation it is not always possible to identify the typical anatomical distribution of germ cells or Sertoli cells, as mentioned above. Figure 2 illustrates different SSTs loaded with fluo-4AM and viewed under phase contrast microscopy (left panels) and fluorescence microscopy (right panels), at different magnifications. Images correspond to 10× (Fig. 2, A and A′), 20× (Fig. 2, B and B′), and 40× (Fig. 2, C and C′) magnifications.
FIG. 2.
Images of seminiferous tubule slices obtained at different magnifications. A–C) Three SSTs observed with phase contrast microscopy at ×10, ×20, and ×40 magnifications. A′–C′) Fluorescence images of the same SST fields after incubation with fluo-4AM. In all images the seminiferous tubules were cut transversally. Bars (top to bottom) = 160, 80, and 40 μm.
Germ Cells Are Viable and Display Spontaneous Ca2+ Oscillations in the SST
Given the anatomical complexity of the SST preparation, it is convenient to mention that for the selection of germ cells in the analysis of our experiments we used criteria such as cell size and shape: the size of round and condensing spermatids is between 8 and 10 μm and they are spherical and elongated respectively, whereas pachytene spermatocytes are spherical and 12–16 μm in size [35]; these parameters were used with the purpose of evaluating the responses of germ cells exclusively. In this regard, it should be mentioned that only 3% of the cell population in the adult SST corresponds to Sertoli cells [35]. Thus it is reasonable to assume that the analysis performed in this study corresponds preponderantly to the activity of male germ cells.
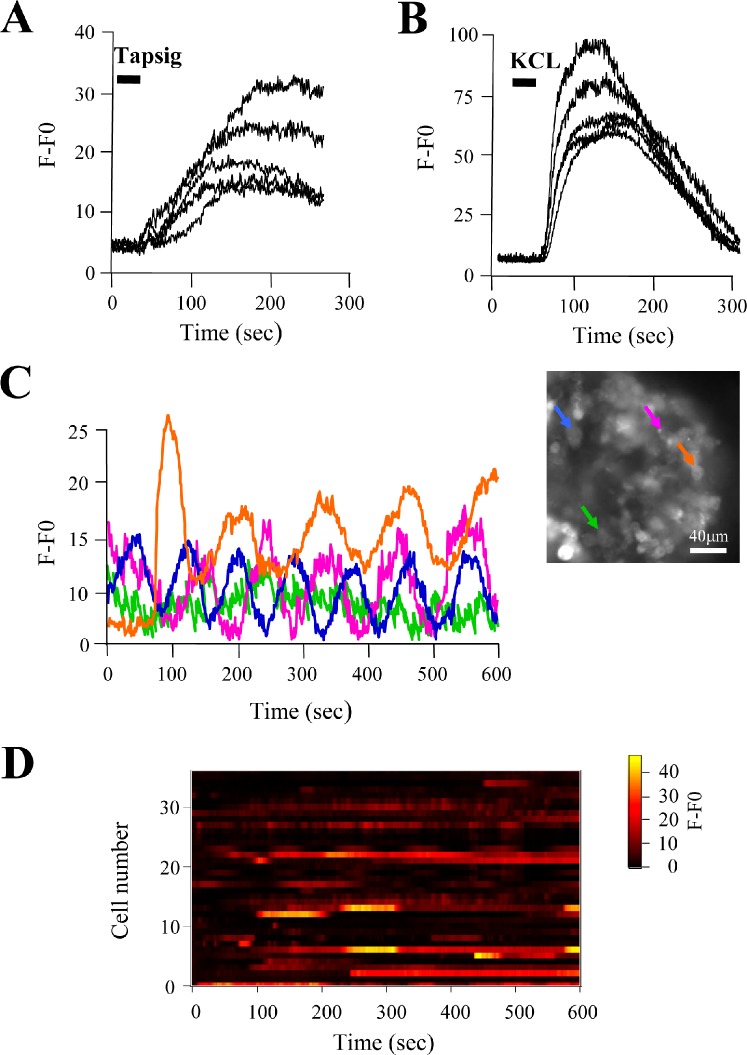
Manipulation and cutting of seminiferous tubules during slice processing could damage germ cells. To test for germ cell viability in the slice, we used two strategies. First, we applied thapsigargin, an ATPase blocker; this drug raises [Ca2+]i by preventing the cell from pumping Ca2+ into the endoplasmic reticulum, causing store depletion. Figure 3A shows that spermatogenic cells responded to thapsigargin application (10 μM) with a sustained [Ca2+]i rise. Thapsigargin promoted a [Ca2+]i increase in 87.3% ± 2.7% of cells analyzed (n = 4 slices, 68 cells). Next, we used a membrane depolarization protocol, which consists in the application of saline containing 120 mM KCl into the bath. This procedure is useful to test the viability of cells because it opens CaV3 channels and/or other voltage-dependent channels and increases the [Ca2+]i. This treatment induced a transient [Ca2+]i increase in 88.1% ± 2.4% of the germ cells studied (n = 4 slices, 48 cells; Fig. 3B). These results suggest that the spermatogenic cells remain in a suitable physiological condition in the SST preparation.
FIG. 3.
Germ cells are viable in the SST and display spontaneous Ca2+ oscillations. Ca2+ recordings from germ cells in the SST corresponding to the addition of 10 μM thapsigargin (A) or 120 mM KCl (B). C) Fluorescence traces obtained from the four cells indicated by colored arrows in the still image (right) from Supplemental Movie S1 (for illustrative purposes the movie was reduced to show one frame every 10 sec and was accelerated to 10 frames per second). Germ cells displayed spontaneous Ca2+ fluctuations. D) Multicell plot showing fluorescence changes in pseudocolor scale corresponding to spontaneous Ca2+ oscillations obtained from 34 germ cells in an SST during 10 min of recording.
Other cell types, such as neurons and endocrine cells, display spontaneous [Ca2+]i oscillations under basal, resting conditions. Oscillations are rhythmic cytoplasmic Ca2+ transients that in some tissues are related to secretion (reviewed in [17, 36]). In Linfocite T cells, Ca2+ oscillations have been related to transcription regulation [37]. The occurrence of [Ca2+]i oscillations has not been explored in germ cells before. To evaluate the presence of spontaneous [Ca2+]i fluctuations in spermatogenic cells, we conducted long-lasting (10 min) Ca2+ imaging protocols under nonstimulated conditions (baseline recordings). Interestingly, approximately 65% ± 6.8% of the germ cells analyzed (n = 10 slices, 386 cells) in SSTs displayed spontaneous Ca2+ oscillations of different frequency and amplitude (Fig. 3C); an example of this activity is shown in Supplemental Movie S1. To detect spontaneous Ca2+ fluctuations in several germ cells, we used a macro designed to plot fluorescence intensity changes corresponding to [Ca2+]i variations of dozens of individual cells. These plots allowed us to identify germ cells displaying spontaneous [Ca2+]i changes (Fig. 3D). In some cases these spontaneous [Ca2+]i fluctuations occurred simultaneously in several germ cells (see below).
Spontaneous Ca2+ Oscillations Are Dependent on Extracellular Ca2+ and Are Partially Inhibited by CaV3 Blockers
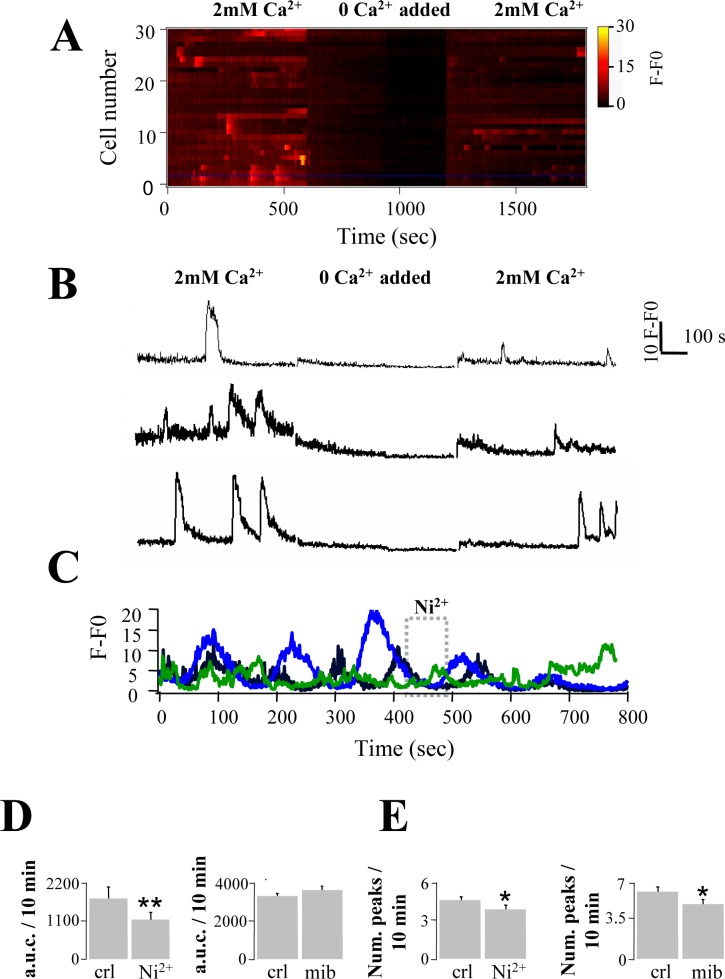
Given that rodent germ cells express CaV3 channels, which allow the entry of extracellular Ca2+ into the cytoplasm of cells [12, 15], we decided to explore the possible participation of these channels in the spontaneous Ca2+ oscillations displayed by germ cells in the SST. Recordings were made first for 10 min in a physiological solution containing 2 mM Ca2+, and then the saline was switched for a solution containing no added CaCl2 during 10 min (Fig. 4A, middle panel). Finally, the slice was perfused again with physiological saline with 2 mM external Ca2+ and the recording continued for 10 more min. The multicell plot (Fig. 4A) shows fluorescence fluctuations corresponding to Ca2+ oscillations displayed under basal conditions by more than 30 individual germ cells in an SST. These spontaneous Ca2+ fluctuations were practically abolished when the physiological solution was replaced with the solution without added Ca2+. This behavior was observed on 81% ± 9.7% of the analyzed cells (n = 4235 cells). Restitution to normal 2 mM external Ca2+ promoted partial recovery of spontaneous Ca2+ oscillations in 57.4% ± 5.0% of cells. Figure 4B shows recordings corresponding to three individual cells from the multicell plot in Figure 4A. Altogether these results suggest that Ca2+ entry is at least partially responsible for the spontaneous Ca2+ oscillations on SST germ cells.
FIG. 4.
Spontaneous Ca2+ oscillations are dependent on extracellular Ca2+ and are partially inhibited by a T type Ca2+ channel blocker. A) Multicell plot of spontaneous fluorescence changes in pseudocolor scale corresponding to Ca2+ fluctuations of germ cells in saline containing 2 mM Ca2+, no Ca2+ added, and 2 mM Ca2+ readdition. B) Individual Ca2+ recordings of three cells included in the multicell plot shown in A. In these cells the spontaneous Ca2+ fluctuations are completely abolished in the absence of extracellular Ca2+ and partially recovered after 2 mM Ca2+ addition. C) Ca2+ recordings of three cells displaying spontaneous Ca2+ oscillations. The amplitude of two of them is reduced after Ni2+ application. Quantitation of the Ni2+ and mibefradil (mib) effect on the area under the curve (D) and on the frequency (E) of spontaneous Ca2+ oscillations: asterisk denotes statistically significant differences, with *P < 0.05 and **P = 0.001, using a Student t-test (n = 5 independent experiments for each condition).
Considering that CaV3 is the main type of Ca2+ channel involved in Ca2+ entry into germ cells [12, 15], we evaluated the effect of Ni2+ and mibefradil, two CaV3 channel blockers, on spontaneous [Ca2+]i oscillations of SST germ cells. As shown in Figure 4C, the application of NiCl2 reduces the amplitude of [Ca2+]i oscillations in two out of the three cells shown. Figure 4D summarizes the reduction caused by Ni2+ (200 μM) and mibefradil (10 μM) in the area under the curve in a 10-min period of [Ca2+]i oscillations: Ni2+ reduced the area under the curve significantly from 1754.5 ± 306.8 to 1145.9 ± 224.6 (P < 0.01), whereas mibefradil did not (control, 3256 ± 156, vs. treatment, 3568 ± 256, P > 0.05). However, when we evaluated the effect of the of Ca2+ channel blockers on the number of [Ca2+]i fluctuations, we found that both Ni2+ and mibefradil diminished the frequency of [Ca2+]i peaks recorded over a 10-min interval compared to the control (from 4.7 ± 0.26 to 3.98 ± 0.34, P < 0.05, and 6.0 ± 0.40 to 4.9 ± 0.41, P < 0.05, respectively). The differential effect observed with both Ca2+ channel blockers could be because of the fact that mibefradil has other targets in testis and sperm that include Catsper [38, 39] and K+ channels [40]. Also, recently it has been shown that mibefradil can elevate basal [Ca2+]i levels in human sperm [41].
Acutely Dissociated Germ Cells Also Display Spontaneous Ca2+ Oscillations
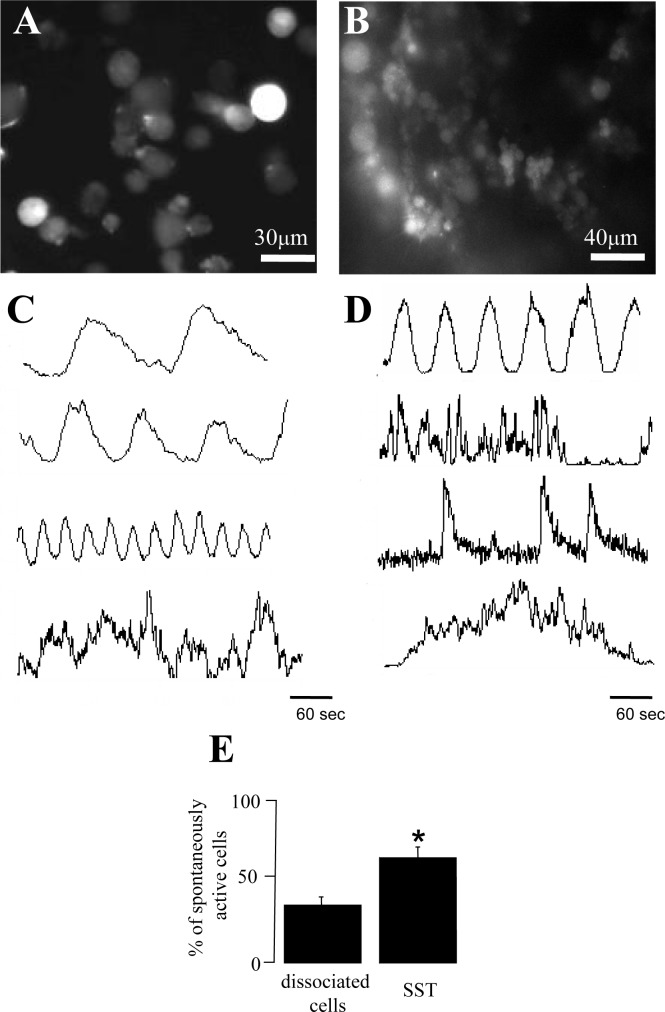
We then investigated if the Ca2+ oscillations were a property of germ cells in their natural environment inside the tubules or if they were also present in dissociated cells. For this purpose we conducted long-lasting Ca2+ imaging records from freshly dissociated germ cells loaded with fluo-4 AM (Fig. 5A). The acquisition protocol was identical to the one applied for SSTs. We found that the majority of freshly dissociated germ cells also display spontaneous Ca2+ oscillations in a sinusoidal Ca2+ pattern (79.66% ± 3.6%), although some (20.34% ± 3.16%) cells showed other Ca2+ fluctuation patterns (see Supplemental Movie S2 and Fig. 5C). On the other hand, only 21% ± 7.7% of germ cells in the SST exhibited low-frequency Ca2+ oscillations with a sinusoidal pattern, similar to what is observed in freshly dissociated cells. Most germ cells in the SST (78.9% ± 7.7%) displayed Ca2+ fluctuation patterns with complex frequency dynamics (n = 10 slices, 437 cells). Some of these complex patterns are illustrated in Figure 5D.
FIG. 5.
Spontaneous Ca2+ fluctuation patterns are different in acutely dissociated germ cells and in SSTs. Fluorescence image obtained from freshly dissociated germ cells (see Supplemental Movie S2; the movie was reduced to show one frame every 10 sec and was accelerated to 10 frames per second) (A). Fluorescence image of germ cells in the SST preparation loaded with fluo-4 AM (B). Examples of spontaneous Ca2+ oscillations displayed by freshly dissociated germ cells (C) or by germ cells in an SST (D). Percentage of spontaneous Ca2+ fluctuations in germ cells freshly dissociated versus the SST preparation: asterisk denotes significant differences (*P = 0.008, Student t-test; dissociated germ cells: n = 5, 107 cells; SST: n = 10, 349 cells).
To explore a possible correlation between Ca2+ oscillation patterns and the stage of germ cell differentiation, we identified three developmental stages in the SST: pachytene, round, and condensing spermatids. Nonetheless, after careful examination of the Ca2+ fluctuation patterns, we were unable to find a clear correlation among germ cell type and Ca2+ fluctuations (data not shown). Next, we compared if intact germ cells in the SST and freshly dissociated germ cells have the same propensity to generate spontaneous Ca2+ oscillations. The results are shown in Figure 5E. As these graphs show, the percentage of germ cells showing spontaneous Ca2+ fluctuations is higher when cells are in the SST preparation (65.5% ± 6.8%, n = 10 slices, 349 cells) than when cells are dissociated (36.1% ± 4.5%, n = 5107 cells); differences are statistically significant (P = 0.008, t-test).
Spontaneous Ca2+ Oscillations Occur Synchronously in Clusters of Germ Cells
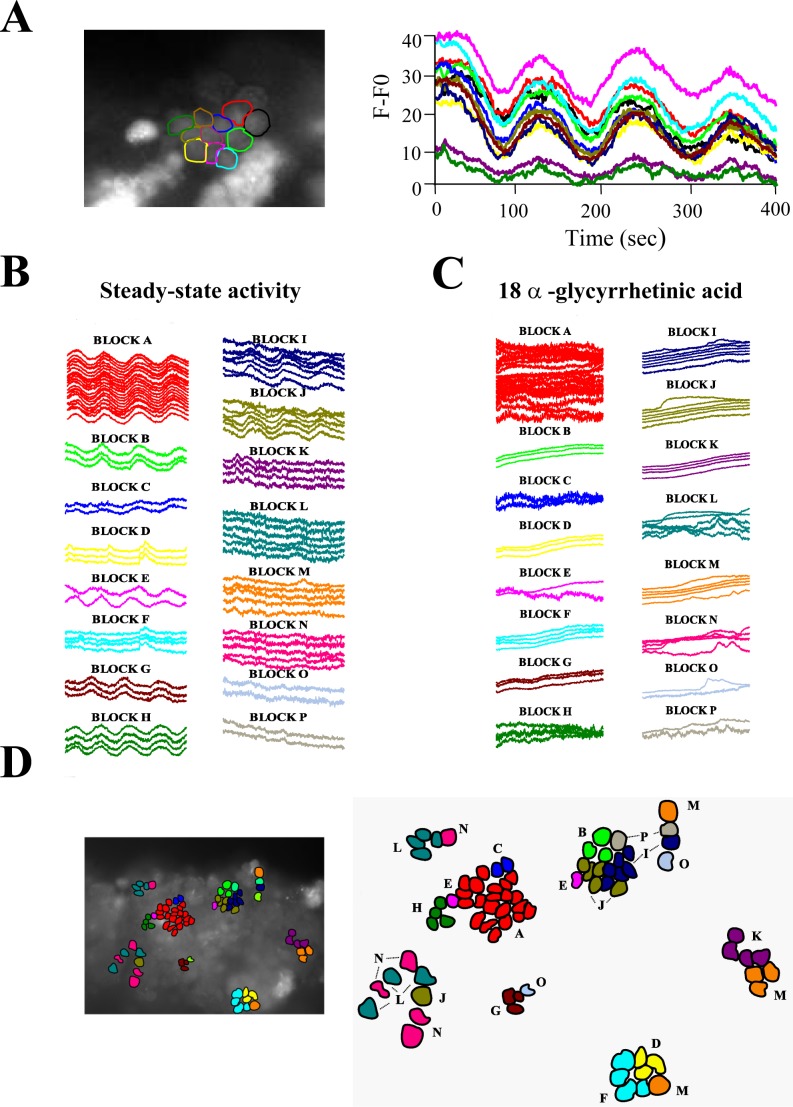
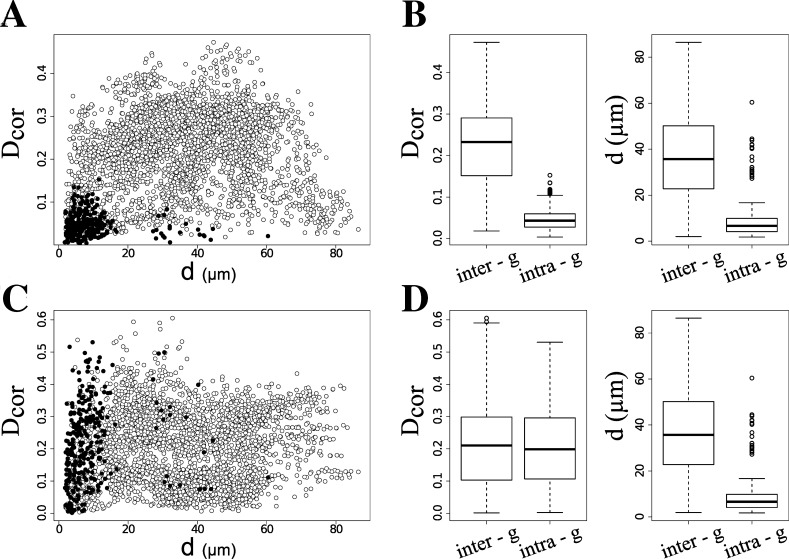
The anatomical and functional preservation of cells in the SST allowed recording some germ cells in clusters that displayed spontaneous synchronized Ca2+ fluctuations (see Fig. 6A). Next, we decided to mathematically evaluate the presence of “coupled” or synchronous spontaneous Ca2+ fluctuations between neighboring cells that has been observed in other tissues. To identify putative groups of cells displaying coupled steady-state Ca2+ fluctuations, a PCA was performed on all SST cells that presented spontaneous [Ca2+]i oscillations. We selected 16 groups of correlation based on a correlation distance Dcor < 0.15 (their spatial proximity on a correlation space defined by the three principal components (PC1, PC2, and PC3; Supplemental Fig. S1A). Single cell [Ca2+]i recordings were organized according to the groups identified (Fig. 6B), and mapped to their physical loci on the SST preparation (Fig. 6C). Interestingly, the cells that showed coupled Ca2+ activity are depicted as blocks of correlation in Figure 6, B and C. Figure 7A shows the physical distance on the SST preparation plotted against the correlation distance Dcor between single pairs of cells experiencing coupled Ca2+ fluctuations (black dots) or uncoupled Ca2+ fluctuations (white dots). The cells showing coupled Ca2+ fluctuations were closer in the SST preparation than the uncoupled cells (P < 0.001, Wilcoxon test). Similar results were obtained with at least three independent SST preparations (data not shown).
FIG. 6.
Clustered germ cells in SST preparations display spontaneous coupled Ca2+ oscillations. A) Left, fluorescence image obtained from a cluster of germ cells displaying spontaneous, synchronized Ca2+ oscillations (see Supplemental Movie S3; the movie was reduced to show one frame every 10 sec and was accelerated to 10 frames per second). Right, color traces correspond to the individual Ca2+ recording of each cell indicated with a colored outline. B) Single Ca2+ time series of SST cells experiencing steady-state Ca2+ fluctuations classified according to the groups of correlation identified by PCA (see Materials and Methods and Supplemental Fig. S2). Each trace corresponds to the normalized fluorescence of an SST cell during 10 min. C) Selected regions occupied by cells displaying synchronous steady-state Ca2+ changes on an SST preparation. The inset shows a fluo-4 AM fluorescence image of an SST with the regions occupied by the selected cells. The color code is the same as in A and indicates the groups of correlation identified. D) Single Ca2+ time series of SST cells after treatment with AGA, organized and colored according to the groups of correlation identified in B showing alterations and/or uncoupling of Ca2+ oscillations.
FIG. 7.
A GJ blocker disrupts the Ca2+ activity coupling of SST germ cells. A, C) Physical distance (d) on the SST against the correlation distance (Dcor) between single pairs of clustered cells (black dots), and between pairs of cells of different clusters (white dots) experiencing steady-state Ca2+ activity (A) and after exposure to AGA (C). B, D) Overall correlation (left) and physical distance (right) between pairs of cells of different correlation clusters (intergroup distance), before (B) and after AGA treatment (D). Each box contains 50% of values, the inner lines indicate the median, and the error bars the define 95% outliers.
In summary, PCA analysis of spontaneous Ca2+ oscillations of SST germ cells reveals coupling of Ca2+ dynamics between clustered cells in physical contact, as shown in Supplemental Movie S3, suggesting functional coupling via local communication.
A GJ Blocker Uncouples the Ca2+ Fluctuations of SST Germ Cell Clusters
In the seminiferous tubules, interacting Sertoli-Sertoli and Sertoli-germ cells express functional GJs [28, 42]. The blocker AGA has been used to suppress electrical coupling between neighboring cells. This compound also decreases the diffusion of a fluorescent dye in the seminiferous tubules due to GJ coupling [29]. We used AGA to investigate if the synchronization of Ca2+ fluctuations observed is mediated by GJs. An SST preparation displaying synchronization of Ca2+ fluctuations (as described earlier) was incubated with 10 μM AGA during 10 min. PCA analysis revealed that some of the clusters identified prior to treatment became uncoupled after AGA treatment (compare Supplemental Fig. S2, A and B, and Fig. 6, B and D, blocks A, C, E, H, L, and N–P). However, other SST cells showed a steady [Ca2+]i rise and a false “coupling” following AGA treatment, probably reflecting impairment of Ca2+ homeostasis in neighboring germ cells (Fig. 6D, blocks B, D, F, G, I–K, and M). The SST cells whose [Ca2+]i increased steadily over time formed a new group of correlation (Fig. S1B; Fig. 6D, blocks B, D, F, G, I–K, and M). The plot of the physical distance (d) against Dcor between single pairs of SST clustered cells showed that some of them missed their synchronous Ca2+ fluctuations but, obviously, not their physical proximity (Fig. 6D, blocks A, C, E, H, L, and N–P; Fig. 7C). Some other SST cells remained coupled (Fig. 7C) but exhibited different Ca2+ dynamics (Fig. 6D, blocks B, D, F, G, I–K, and M). However, the overall Ca2+ coupling between germ cells in the SST was impaired by AGA: their overall intragroup correlation distance increased (compare Figs. 7B and 6D, left; P < 0.001, Wilcoxon test), but not their physical proximity (Fig. 7, B and D, right).
T Promotes an Increase in [Ca2+]i in Some Cells in the SST
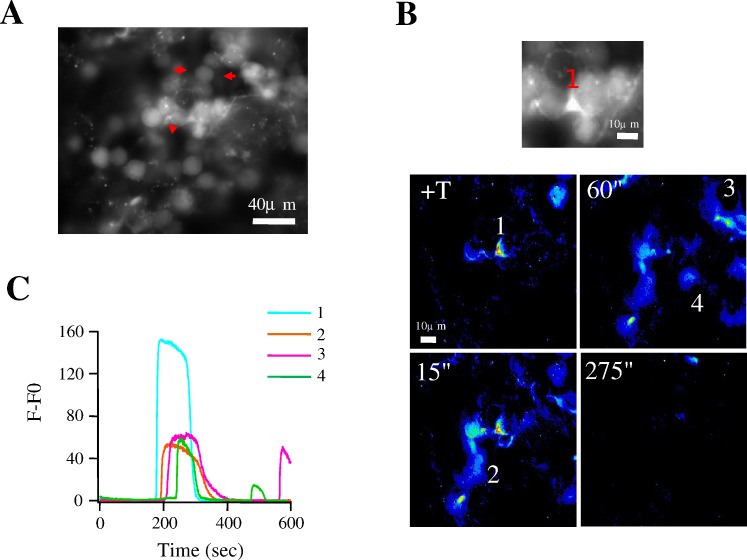
T is a hormone crucial for spermatogenesis that exerts its effects indirectly via an androgen receptor localized in Sertoli cells (reviewed in [43]). These cells are essential for the maintenance and development of spermatogenesis, because they give support and nutrition to germ cells. Sertoli cells are coupled to each other by GJs and this coupling is fundamental for spermatogenesis. Considering that the SST preparation allows partial conservation of the tissue architecture, we explored the possibility of identifying functional Sertoli cells in our preparation by looking at their specific Ca2+ signals. Previous studies using freshly dissociated Sertoli cells and primary culture of seminiferous tubules reported that nanomolar concentrations of T promote [Ca2+]i elevations in Sertoli cells [5, 44]. Germ cells and Sertoli cells are intermingled, which makes the identification of the latter difficult in our preparation. For this reason, and taking advantage of the fact that the expression of T receptors is low in germ cells [45], we applied this hormone to SSTs during Ca2+ recordings to try to identify Sertoli cells. In three of these experiments we were able to detect elongated cells intermingled with germ cells, an anatomical characteristic compatible with Sertoli cells, which responded to T (100 nM) application with a [Ca2+]i increase. An example of the response of one of these cells is shown in Figure 8A and Supplemental Movie S4. Here, the red arrows indicate a cytoplasmic prolongation of a putative Sertoli cell located between three germ cells. Figure 8B shows a sequence of fluorescence images obtained after T application: the first panel exemplifies one cell (1) responding to T application with a Ca2+ increase (+T). In the second panel (15 sec later) the Ca2+ signal spreads to another two cells (2) probably corresponding to germ cells. After 60 sec, the Ca2+ signal propagates, apparently through cytoplasmic processes that could be part of Sertoli cells surrounding germ cells (3, 4). Finally, the last panel (275″) shows that Ca2+ signals from all participating cells in the field terminated. Figure 8C illustrates the individual Ca2+ responses promoted by T application to cells labeled 1–4 in Figure 8B. These records suggest that Ca2+ signals stimulated by this hormone are slow, transient, and initiated in Sertoli cells, then spread to other neighboring cells that could include both germ cells and other Sertoli cells.
FIG. 8.
T promotes Ca2+ responses in elongated cells into the SST. A) Fluorescence image of an SST. Elongated structures can be seen between germ cells (shown by red arrows). B) As shown in Supplemental Movie S4, T application (100 nM) promotes an immediate Ca2+ increase in one cell, illustrated in the upper panel (1), also shown in the first pseudocolored panel (+T); the response is propagated to other cells after 15 (15″) and 60 sec (60″); the Ca2+ increase disappears on these cells after 275 sec (275″) of T application. The movie was reduced to show one frame every 10 sec and was accelerated to 10 frames per second. C) Transitory Ca2+ responses promoted by T in cells 1, 3, and 4 in the pseudocolored images in B.
DISCUSSION
Ca2+ oscillations are generated by periodic Ca2+ influx from the extracellular medium and/or Ca2+ release from intracellular Ca2+ stores. These Ca2+ increases result from the orchestrated activity of ion channels, transporters, and pumps on the plasma membrane and the ER, which coordinately regulate Ca2+ homeostasis in the cytoplasm of cells. Ca2+ oscillations are involved in the regulation of several cellular functions, including secretion, development, differentiation, and fertilization [46, 47]. Previous studies reported, not surprisingly, that male germ cells possess their own mechanisms to regulate cytoplasmic Ca2+, which include voltage-gated Ca2+ channels and intracellular channels, InsP3R and ryanodine receptors [9, 12, 15]. However, few studies report [Ca2+]i dynamics in spermatogenic cells [16, 48–51]. In this work we implemented for the first time a technique that allows the study of [Ca2+]i dynamics on dozens of germ cells in a context that preserves the architecture of the native tissue. We provide evidence that the spontaneous Ca2+ fluctuations in germ cells depend, at least in part, on Ca2+ entry through CaV3 channels. The Ca2+ oscillation patterns exhibited by freshly dissociated germ cells are different from those displayed by these cells in the acute SST preparation. Our results have demonstrated that spontaneous Ca2+ fluctuations in germ cells can occur synchronously in clusters composed of up to 20–21 cells in the SST (Fig. 6B). These clusters display the Ca2+ oscillation pattern for long periods of time (at least 10 min). In contrast, other cells display asynchronous spontaneous Ca2+ oscillations never involving other cells during the recording. Our experiments suggest that GJs could be mediating the synchronization of spontaneous Ca2+ oscillations in germ cells. However, because the GJ blocker (AGA) affects synchronization in some clusters but not in others, additional mechanisms such as IBs should be invoked to explain the synchronization phenomena.
Additionally, the SST preparation allowed us to record Ca2+ responses stimulated by T application in cells that functionally and anatomically resemble Sertoli cells. All these findings indicate that SST is an ideal preparation to begin approaching the study of functional cell-cell interactions during germ cell differentiation in the testis seminiferous tubules.
Ca2+ oscillations in germ cells are involved in many important cell functions. The development and differentiation of oocytes in the ovary is a relevant example (reviewed in [52]). Immature oocytes display spontaneous Ca2+ oscillations [45], which are present in ∼70% of oocytes released from the antral or preantral follicles [53, 54]. Notably, the presence of Ca2+ oscillations in male germ cells has not been previously described. In this work we found that 65% of spermatogenic cells in SST generate spontaneous Ca2+ oscillations, whereas only 36% of dissociated cells do so. This could be an indication of cell damage or removal of cell-to-cell communication with neighboring cells during dissociation. Spermatogenic cells displayed regular and irregular spontaneous Ca2+ fluctuations ranging from 0.1 to 2 spikes/sec (60-fold more frequent than oocytes [53]). In oocytes, the patterns of spontaneous Ca2+ oscillations depend on the stage of oocyte maturation [53]. Nonetheless, we did not observe significant differences of Ca2+ oscillation patterns at different stages of spermatogenic cell maturation, at least in the developmental range from pachytene spermatocytes to condensing spermatids. Also, we observed a higher proportion (78.9%) of irregular signaling patterns in germ cells in the SST, which could indicate more complex Ca2+ signaling events, possibly due to interactions with different cells type in the tissue.
Ca2+ oscillations have been related to the control of gene expression during cell differentiation. An example of this was shown using microarray studies on cultured mouse cerebellar granular cells exposed to depolarized conditions. In this study, membrane potential depolarization, linked to Ca2+ entry, was related to the induction of many genes implicated in cell proliferation, differentiation, migration, and neurite growth of immature granule cells [55]. In oocytes it has also been shown that a [Ca2+]i rise is important during differentiation by suppressing the meiotic arrest and leading to meiosis progress [56]. Although it is difficult to predict which role Ca2+ oscillations may have on germ cell physiology and development, their presence could impact gene regulation, transcription, and/or structural and functional regulation during spermatogenesis.
Several research groups have made technical efforts to mimic the native testis environment, using mainly variants of cellular cocultures to perform functional studies [57–59]. However, only the use of testis organ culture has recently allowed the successful development of mammalian spermatogenesis in vitro [60], indicating that good tissue preservation is fundamental for appropriate male germ cell differentiation and maturation. Interestingly, we observed that spontaneous Ca2+ oscillations in male germ cells occur synchronously in the SST preparation, suggesting that functional cell-cell communication is important during spermatogenesis. The effect of AGA on spontaneous Ca2+ oscillations is consistent with the participation of GJs in spermatogenic cell coupling. This result is in agreement with immunodetection of connexin 43 in spermatogenic cells [40]. The IBs are evolutionarily conserved and allow cytoplasm sharing among spermatogenic cells during the synchronous cell divisions in the seminiferous tubules [30]. IBs are 1–3 μm in diameter [31] and form syncytial clusters resulting from incomplete cytokinesis. These IBs allow the movement of many cellular components, including cytoplasmic materials and even organelles [61]. Therefore, the free passage of Ca2+ ions is not difficult to imagine and could explain the presence of functional clusters displaying synchronous Ca2+ oscillations, as we observed in this study. Electron microscopy reports in rodent germ cells have detected syncytia composed of 2–100 spermatogenic cells [62]. In our study, we demonstrated synchronous Ca2+ fluctuations in clusters composed of 2–21 cells. Perhaps we are unable to detect larger clusters simply because the fluorescence signal is collected from a focal plane in the tissue, and we could be missing information from cluster members deeper into the SST. Three-dimensional confocal microscopy may represent an alternative to circumvent this limitation [63]. Also, it is possible that not all spermatogenic cells from a cluster display the same Ca2+ pattern. One can imagine that other elements, such as GJs or paracrine factors, could modify the spontaneous Ca2+ patterns of one part of the cluster, despite the presence of IBs. Thus, we believe that synchronous Ca2+ fluctuations in spermatogenic cells in the SST result from more than one mechanism, including GJs, IBs, or paracrine/autocrine factors. However, independently of the mechanism involved, given that germ cells joined by IBs exhibit synchronous differentiation [30], we hypothesize that Ca2+ diffusion through these structures could participate as a synchronization signal to regulate differentiation during spermatogenesis.
The results obtained in experiments using Ca2+ channel blockers suggest that these channels are at least one of the Ca2+ sources participating in the spontaneous Ca2+ oscillations of spermatogenic cells. These findings coincide with previous reports using electrophysiological [11, 15], immunocytochemical, and molecular techniques [8–10] suggesting the presence of CaV3 channels in spermatogenic cells. As shown here, Ca2+ oscillations are practically abolished in the absence of extracellular Ca2+. However, we cannot rule out the possibility that Ca2+ release from intracellular Ca2+stores mediated by ryanodine and InsP3 receptors also participates in the generation of Ca2+ signals. In fact, the expression of these intracellular receptors has been reported in spermatogenic cells [12, 16].
Spermatogenic tubules are composed mainly of germ and Sertoli cells. The developing germ cells are in close contact with Sertoli cells that nurture them and provide them with structural support. These Sertoli-spermatogenic cell contacts are crucial for spermatogenesis and involve both tight junctions and GJs [28, reviewed by 64]. Branches of Sertoli cell cytoplasm surround the differentiating spermatocytes and spermatids [65], forming a three-dimensional network through which spermatogenesis is modulated via paracrine factors, hormones, proteases, protease inhibitors, and components of the extracellular matrix produced by both Sertoli and germ cells (reviewed in [65]). The Ca2+ responses stimulated by application of T in the SST suggest that Sertoli cells remain functional in the slice, allowing the spreading of Ca2+ waves between networks of Sertoli cells possibly mediated through GJs.
In summary, we demonstrated that the SST is an excellent biological preparation to investigate germ cell function in vitro. In this preparation, intercellular connections remain functional and paracrine/autocrine interactions between the different cell types of the seminiferous tubules continue to take place. Unlike other preparations requiring 12–24 h of stabilization and recovery, the SST preparation allows physiological recordings within 1 h after dissection. This should be relevant if one attempts to preserve physiological responses as reliably as possible. The spontaneous Ca2+ oscillations displayed by spermatogenic cell clusters in the SST attest to their viability and functional state. We believe that SST is a preparation with great potential for future studies on the role of orchestrated [Ca2+]i changes in spermatogenesis.
ACKNOWLEDGMENT
We are grateful to Dr. León Islas for writing Igor-Pro algorithms and to Jorge Carneiro for his comments on the PCA analysis. We also express our gratitude to Jose Luis De la Vega, Yoloxóchitl Sánchez, Shirley Ainsworth, Elizabeth Mata, Marcela Ramírez, Nicolás Jiménez, Diana Millán, and Claudia V. Rivera-Cerecedo for their excellent technical support.
Footnotes
Supported by grants IN227910, IN225406, and IN202212-3 from DGAPA-UNAM; 79763, 9933, 102085, and 128566 from CONACyT (Consejo Nacional de Ciencia y Tecnología, México); and PICSA10-116 from ICyTDF (Instituto de Ciencia y Tecnología del Distrito Federal) to A.H.-C.; and National Institutes of Health grant R01 HD038082-07A1.
REFERENCES
- Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl 2004; 27:335 342 [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747 806 [DOI] [PubMed] [Google Scholar]
- Syed V, Hecht NB. Disruption of germ cell-Sertoli cell interactions leads to spermatogenic defects. Mol Cell Endocrinol 2002; 186:155 157 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003; 4:517 529 [DOI] [PubMed] [Google Scholar]
- Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology 1995; 136:2052 2059 [DOI] [PubMed] [Google Scholar]
- Gorczynska E, Handelsman DJ. The role of calcium in follicle-stimulating hormone signal transduction in Sertoli cells. J Biol Chem 1991; 266:23739 23744 [PubMed] [Google Scholar]
- Loss ES, Jacobus AP, Wassermann GF., Diverse FSH. and testosterone signaling pathways in the Sertoli cell. Horm Metab Res 2007; 39:806 812 [DOI] [PubMed] [Google Scholar]
- Tovey SC, Godfrey RE, Hughes PJ, Mezna M, Minchin D, Mikoshiba K, Michelangeli F. Identification and characterization of inositol 1,4,5-trisphosphate receptors in rat testis. Cell Calcium 1997; 21:311 319 [DOI] [PubMed] [Google Scholar]
- Treviño CL, Santi CM, Beltrán C, Hernández-Cruz A, Darszon A, Lomeli H. Localisation of inositol trisphosphate and ryanodine receptors during mouse spermatogenesis: possible functional implications. Zygote 1998; 6:159 172 [DOI] [PubMed] [Google Scholar]
- Chiarella P, Puglisi R, Sorrentino V, Boitani C, Stefanini M. Ryanodine receptors are expressed and functionally active in mouse spermatogenic cells and their inhibition interferes with spermatogonial differentiation. J Cell Sci 2004; 117:4127 4134 [DOI] [PubMed] [Google Scholar]
- Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol. Pharmacol 1998; 53:1104 1111 [PubMed] [Google Scholar]
- Treviño CL, Felix R, Castellano LE, Gutierrez C, Rodriguez D, Pacheco J, Lopez-Gonzalez I, Gomora JC, Tsutsumi V, Hernandez-Cruz A, Fiordelisio T, Scaling AL. Darszon A. Expression and differential cell distribution of low-threshold Ca2+ channels in mammalian male germ cells and sperm. FEBS Lett 2004; 563:87 92 [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation and function of spermatozoa. Physiol Rev 2011; 91:1305 1355 [DOI] [PubMed] [Google Scholar]
- Benoff S. Voltage dependent calcium channels in mammalian spermatozoa. Front Biosci 1998; 3:D1220 1240 [DOI] [PubMed] [Google Scholar]
- Liévano A, Santi CM, Serrano CJ, Treviño CL, Bellvé AR, Hernández-Cruz A, Darszon A. T-type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett 1996; 388:150 154 [DOI] [PubMed] [Google Scholar]
- Berrios J, Osses N, Opazo C, Arenas G, Mercado L, Benos DJ, Reyes JG. Intracellular Ca2+ homeostasis in rat rouns spermatids. Biol Cell 1998; 90:391 398 [PubMed] [Google Scholar]
- Stojilkovic SS. Pituitary cell type-specific electrical activity, calcium signaling and secretion. Biol Res 2006; 39:403 23 [DOI] [PubMed] [Google Scholar]
- Hellman B, Gylfe E, Grapengiesser E, Lund PE, Berts A. Cytoplasmic Ca2+ oscillations in pancreatic beta-cells. Biochim Biophys Acta 1992; 1113:295 305 [DOI] [PubMed] [Google Scholar]
- Malgaroli A, Meldolesi J. [Ca2+] oscillations from internal stores sustain exocytotic secretion from the chromaffin cells of rat. FEBS Lett 1991; 283:169 172 [DOI] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Martinez-Fuentes AJ, Stojilkovic SS. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem 2001; 276:33840 33846 [DOI] [PubMed] [Google Scholar]
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, et al. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A 2005; 102:16880 16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod 2004; 71:375 391 [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci 2010; 365:1621 1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 2003; 83:1359 1400 [DOI] [PubMed] [Google Scholar]
- Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 2007; 94:120 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batias C, Siffroi JP, Fénichel P, Pintis G, Segretain D. Conexin 43 gene expression and regulation in the rodent seminiferous epithelium. J Histochem Cytochem 2000; 48:793 805 [DOI] [PubMed] [Google Scholar]
- Bravo-Moreno JF, Díaz-Sánchez V, Montoya-Flores JG, Lamoyi E, Saénz CJ, Pérez-Armendáriz ME. Expression of conexin43 in mouse Leydig, Sertoli, and germinal cells at different stages of postnatal development. Anat Rec 2001; 264:13 24 [DOI] [PubMed] [Google Scholar]
- Decrouy X, Gasc JM, Pointis G, Segretain D. Functional characterization based gap junctions during spermatogenesis. J Cell Physiol 2004; 200:146 154 [DOI] [PubMed] [Google Scholar]
- Gilleron J, Carette D, Durand P, Pointis G, Segretain D. Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int J Biochem Cell Biol 2009; 41:1381 1390 [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol 1959; 5:453 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod 1971; 4:195 215 [DOI] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 4: intercellular bridges, mitochondria, nuclear envelope, apoptosis, ubiquitination, membrane/voltage-gated channels methylation/acetylation, and transcription factors. Microsc Res Tech 2010; 73:364 408 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.r-project.org/
- Jolliffe IT. Principal Component Analysis , 2nd ed. Springer Series in Statistics. New York: Springer; 2002:487 [Google Scholar]
- Bellvé AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol 1993; 225:84 113 [DOI] [PubMed] [Google Scholar]
- Low JT, Shukla A, Thorn P. Pancreatic acinar cell: new insights into the control of secretion. Int J Biochem Cell Biol 2010; 42:1586 1589 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998; 392:933 936 [DOI] [PubMed] [Google Scholar]
- Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA 2001; 98:12527 12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JL, O'Doherty AM, Wang S, Zheng S, Sanders KM, Yan W. Catsper 3 and Catsper 4 encode two cation channel-like proteins exclusively expressed in testis. Biol Reprod 2005; 73:1235 1242 [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA 2007; 104:7688 7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Flores-V, Picaso-Juárez G, Hernández-Rueda Y, Darszon A, Gonzalez-Martínez MT. Sodium influx induced by external calcium chelation decreases human sperm motility. Hum Reprod. 2011; 26:2626 2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley MS, Tan IP, Roy C, Sáenz JC. Cell-, age- and stage-dependent distribution of connexin 43 gap junctions in testes. J Cell Sci 1992; 103:81 96 [DOI] [PubMed] [Google Scholar]
- Silva FR, Leite LD, Wassermann GF. Rapid signal transduction in Sertoli cells. Eur J Endocrinol 2002; 147:425 433 [DOI] [PubMed] [Google Scholar]
- Lying FM, Jones GR, Rommerts FF. Rapid androgen actions on calcium signaling in rat Sertoli cells and two human prostatic cell lines similar biphasics responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod 2000; 63:736 47 [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 1994; 135:1227 34 [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem 1992; 267:11196 11201 [PubMed] [Google Scholar]
- Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol 2009; 297:58 72 [DOI] [PubMed] [Google Scholar]
- Reyes JG, Herrera E, Lobos L, Salas K, Lagos N, Jorquera RA, Labarca P, Benos DJ. Dynamics of intracellular calcium induced by lactate and glucose in rat pachytene spermatocytes and round spermatids. Reproduction 2002; 123:701 710 [DOI] [PubMed] [Google Scholar]
- Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+ levels regulate BCL-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem 2006; 281:2133 2143 [DOI] [PubMed] [Google Scholar]
- Treviño CL, De la Vega-Beltrán JL, Nishigaki T, Felix R, Darszon A. Maitotoxin potently promotes Ca2+ influx in mouse spermatogenic cells and sperm, and induces the acrosome reaction. J Cell Physiol 2006; 206:449 456 [DOI] [PubMed] [Google Scholar]
- Reyes JG, Osses N, Knox M, Darszon A, Treviño CL. Glucose and lactate regulate maitotoxin-activated Ca2+ entry in spermatogenic cells: the role of intracellular [Ca2+]. FEBS Lett 2010; 584:3111 3115 [DOI] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 2008; 315:257 279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development 1994; 120:3507 3517 [DOI] [PubMed] [Google Scholar]
- Gomes JE, Pesty A, Gouveia-Olveira A, Cidadao AJ, Plancha CE, Lefèvre B. Age and gonadotropins control Ca2+-spike acquisition in mouse oocytes isolated from early preantral follicles. Int J Dev Biol 1999; 43:839 842 [PubMed] [Google Scholar]
- Sato M, Suzuki K, Yamazaki H, Nakanishi S. A pivotal role of calcineurine signaling in development and maturation of posnatal cerebellar granule cells. Proc Natl Acad Sci U S A 2005; 102:5874 5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni R, Gualtieri R, Talevi R, Tosti E. Calcium and other ion dynamics during gamete maturation and fertilization. Theriogenology 2007; 68:S156 164 [DOI] [PubMed] [Google Scholar]
- Godet M, Sabido O, Gilleron J, Durand P. Meiotic progression of rat spermatocytes requires mitogen-activated protein kinases of Sertoli cells and close contacts between the germ cells and the Sertoli cells. Dev Biol 2008; 315:173 188 [DOI] [PubMed] [Google Scholar]
- Sá R, Neves R, Fernandes S, Alves C, Carvalho F, Silva J, Cremades N, Malheiro I, Barros A, Sousa M. Cytological and expression studies and quantitative analysis of the temporal and stage-specific effects of follicle-stimulating hormone and testosterone during cocultures of the normal human seminiferous epithelium. Biol Reprod 2008; 79:962 975 [DOI] [PubMed] [Google Scholar]
- La Salle S, Sun F, Handel MA. Isolation and short-term culture of mouse spermatocytes for analysis of meiosis. Methods Mol Biol 2009; 558:279 297 [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011; 471:504 508 [DOI] [PubMed] [Google Scholar]
- Ventelä S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol Biol Cell 2003; 14:2768 2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Hugenholtz AD. The arrangement of germ cells in the rat seminiferous tubule: an electron-microscope study. J Cell Sci 1975; 19:487 507 [DOI] [PubMed] [Google Scholar]
- Corkidi G, Taboada B, Wood CD, Guerrero A, Darszon A. Tracking sperm in three-dimensions. Biochem Biophys Res Commun 2008; 373:125 129 [DOI] [PubMed] [Google Scholar]
- Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci 2010; 365:1593 1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Foster DW. Williams Textbook of Endocrinology, 8th ed. Philadelphia: W.B. Saunders Company; 1992: 802 [Google Scholar]