Abstract
OBJECTIVE
Type 1 diabetes is a common chronic childhood disease, and the incidence is increasing globally. Childhood infections are considered a potential environmental trigger of type 1 diabetes. Alternatively, improved hygiene and reduced childhood infections could explain the increase in type 1 diabetes in developed countries. The association of reported illnesses during infancy and later development of islet autoimmunity (IA) were examined in the Diabetes Autoimmunity Study in the Young.
RESEARCH DESIGN AND METHODS
Complete illness interviews through 9 months of age were collected for 1,729 children—1,174 without a family history of type 1 diabetes and 555 with a first-degree relative with type 1 diabetes. Persistent IA was defined as positive antibodies to insulin, glutamic acid decarboxylase, or tyrosine phosphatase on at least two consecutive study visits.
RESULTS
There were 109 children with persistent IA among the 1,729 children with illness records. A greater number of gastrointestinal illnesses were associated with an increased risk of IA, but only among children who were exposed to gluten-containing grains (wheat or barley) either <4 months of age (hazard ratio 1.37 [95% CI 1.22–1.55]; P < 0.0001) or ≥7 months of age (1.12 [1.05–1.19]; P = 0.0005) compared with 4–6 months of age (P for interaction = 0.02). There were no associations of upper respiratory symptoms, respiratory illnesses, or fevers with IA.
CONCLUSIONS
Specific pathogens such as enteroviruses or rotavirus may increase the risk of IA in the presence of existing inflammation induced by diet.
Type 1 diabetes is one of the most common chronic childhood diseases, and the incidence is increasing globally (1,2). This autoimmune disease is characterized by destruction of the insulin-producing β-cells of the pancreas and is preceded by a period of preclinical islet autoimmunity (IA) (3). Type 1 diabetes is strongly influenced by genetics, with increased susceptibility in individuals carrying certain HLA alleles and non-HLA gene variants (4). However, the increasing incidence of type 1 diabetes has been too rapid to attribute to changing genetic risk, and there are likely environmental triggers that influence the risk as well as timing of development of type 1 diabetes. Common theories on environmental triggers include early childhood diet (5–9), stress (10), and viral infections (11). Although there has been some speculation that the development of preclinical IA could be increased by exposure to enteroviral or rotoviral infections, another theory, the hygiene hypothesis, is that reduced microbial exposure and nonspecific infections may increase the risk for type 1 diabetes (12,13).
The hygiene hypothesis has been bolstered by animal studies; disease incidence in NOD mice increases when pups are raised in a pathogen-free environment (14) and decreases when pups are exposed to viral and bacterial pathogens early in life (15). Case-control studies in children with type 1 diabetes have found that infections during the first year of life had a protective effect in the development of type 1 diabetes (16), although a recently published case-control study in the U.K. found no association between infections in early life and subsequent risk of type 1 diabetes (17). However, this study was based on illnesses documented in the child’s medical record and thus did not account for routine illnesses for which the child was not seen by the pediatrician. Studies on maternal illness during pregnancy have found that children whose mothers reported at least one symptom of infection during pregnancy had significantly lower risk of autoimmunity compared with children whose mothers reported no illnesses during pregnancy (18).
Data from prospective studies examining common childhood infections and the risk of IA are lacking, and potential associations between infant dietary exposures such as breast-feeding and introduction of gluten with childhood infections have rarely been studied. Therefore, the objective of this study was to examine infection during the first year of life in association with infant diet and subsequent development of diabetic IA in the Diabetes Autoimmunity in the Young (DAISY) prospective cohort study.
RESEARCH DESIGN AND METHODS
Study participants
The DAISY study is a prospective study located in Denver, Colorado. The purpose of DAISY is to examine the development of type 1 diabetes in a genetically at-risk population. The study consists of two cohorts: the general population (GP) cohort and first-degree relative (FDR) cohort. For recruitment of the GP cohort, the cord blood of >31,000 infants born at St. Joseph Hospital (Denver, CO) during the period from November 1993 to September 2004 was screened for high- or moderate-risk HLA genotypes determined to be associated with an increased risk of IA. Families were excluded if they did not understand English or if their child had a severe congenital malformation or disease. For children who were found to possess the high- or moderate-risk genotypes, families were invited to participate in the follow-up study. In this analysis, 373 children had the highest-risk HLA genotype, and 719 had a moderate-risk HLA genotype. A small number of children with low-risk HLA genotypes for type 1 diabetes were included in the study (n = 82 in the current analysis), the majority of these attributed to having a high-risk HLA genotype for celiac disease, which is also studied in this cohort. The children who were enrolled from the FDR cohort were not selected based on HLA genotype. For this analysis, only children <3 months of age at enrollment who had an FDR were included in the FDR newborn cohort. In the FDR cohort, 56 children had the highest-risk HLA genotype, 203 had a moderate-risk HLA genotype, and 296 had a low-risk HLA genotype.
Genotyping for HLA
Whole-blood samples from newborn umbilical cord samples stored in EDTA were analyzed at Roche Molecular Systems for PCR-based class II genotyping. Children in the GP cohort were categorized into three groups according their odds of developing type 1 diabetes. The high-risk group (odds 1:16) was DRB1*04-DQB1*0302/DRB1*0301-DQB1*0201, and the moderate-risk group (odds 1:75 in non-Hispanic white and 1:230 in Hispanics) was DRB1*04-DQB1*0302/DRB1*04-DQB1*0302 or DRB1*0301/*0301 or DRB1*04,-DQB1*0302/X, where X does not include DRB1*04,DQB1*0302,DRB!*0301,DQB1*0602 or DR2 (19). All other genotypes were classified as low risk.
Study assessments
Children were seen in the clinic at 9, 15, and 24 months of age and yearly after that. Blood samples were drawn at each clinic visit and tested for IA. Of the children enrolled in the study with HLA genotyping, complete illness interviews through 9 months of age were collected for 1,729 children, 1,174 without a family history of type 1 diabetes, and 555 children with an FDR with type 1 diabetes.
This study was approved by the Colorado Multiple Institutional Review Board (Protocol 92-080). Informed consent was obtained from the parents for all children enrolled into the study.
Autoantibody assays
The blood samples drawn at each clinic visit were analyzed for three diabetes-related IA to predict development of type 1 diabetes: glutamic acid decarboxylase (GAD)65, insulin, and IA-2 (20). Autoantibody assays were performed in the laboratory of Dr. George Eisenbarth at the Barbara Davis Center for Childhood Diabetes. Insulin autoantibodies were measured by a microinsulin autoantibody assay with sensitivity of 58%, specificity of 99%, and interassay coefficient of variation of 11% (21). The combined anti-GAD and IA-2 radioassay was performed in duplicate on a 96-well filtration plate, and radioactivity was counted on a TopCount 96-well plate B-counter (PerkinElmer) using a modification of a previously reported method. The levels of both antibodies are expressed as an index: (sample counts per minute [cpm] − negative control cpm)/(positive control cpm − negative control cpm). In the 1995 Immunology of Diabetes Society Workshop, the GAD assay had 82% sensitivity and 99% specificity using the serum from new-onset diabetic patients <30 years of age. The interassay coefficient of variation was 10% (22). All samples with insulin autoantibody, GAD, or IA-2 levels exceeding the 99th percentile and a random 10% of the remaining samples were retested in a blinded manner for quality assurance. The 99th percentile based on testing 198 nondiabetic control subjects aged 0.4–67 years was 0.01 for insulin, 0.032 for GAD, and 0.049 for IA-2. The single highest value for IA-2 among control subjects (0.07) was used as a cutoff for positivity. We defined IA as being positive for one or more autoantibodies at two or more consecutive clinic visits.
Environmental exposure interviews
Interviews were completed with the primary caretaker at 3, 6, and 9 months. Most of these interviews were conducted by telephone, but some were conducted face to face at the clinic visit. The interviews were designed to assess the child’s environmental exposures during the preceding 3-month period, including illness, breast-feeding duration, vitamin or supplement intake, attendance at daycare, and household size and crowding.
The illness portion of the interview was split into two sections: symptoms and diseases. Symptoms included upper respiratory symptoms (runny nose, stuffy nose, cough, cold, sinus infection, and ear infection); gastrointestinal (GI) symptoms (diarrhea and vomiting); and fever (>100°F). Diseases included chicken pox, croup, pneumonia, bronchitis, strep, GI infection, and meningitis. If the parent reported any symptoms, they were prompted for the number of times the child had the symptoms, whether the child was seen by the doctor, the date symptoms began, and a diagnosis. If the parent reported a disease, they were prompted for the age of the child when the disease occurred and if it was diagnosed. Beginning in 1997, the form changed in order to match symptoms with specific illnesses. The parents were asked how many times their child had been sick in the previous 3 months. “Sick” was defined as a condition that made the child unable to participate in normal activities. For this analysis, we examined symptoms from both versions of the questionnaire grouped as upper respiratory symptoms (cough, cold, runny nose, stuffy nose, sinus infection, or ear infection) or GI illness (vomiting or diarrhea), any episode of fever >100°F, and respiratory disease (croup, pneumonia, or bronchitis). We looked at the number of reported symptoms or disease episodes for each child within the first 9 months of life. An indicator variable was used to control for the type of illness survey used in all models examining illnesses.
Infant diet data were collected during telephone or face-to-face interviews with the primary caretaker at 3, 6, and 9 months of age. During each interview, the primary caretaker was asked to report the date of introduction of all foods, including formula, milk, cereals, and other solid foods. Brand name, type, and frequency were recorded for all formulas and cereals. Mothers were asked whether the infant was breast-fed, and the dates of breast-feeding initiation and termination were recorded.
Introduction to cereals was categorized by month of life as early (0–3 months, before the fourth month of life), recommended (4–6 months, >3 months, and up to 6 months of life), and late (≥7 months, >6 months of age), based on 4–6 months as the recommended age for introduction of solid foods, including cereals, according to the American Academy of Pediatrics (23). Cereal grains were grouped as gluten-containing (wheat and barley), gluten-containing and rice (wheat, barley and rice), and all grains (wheat, barley, rice, and oats).
Statistical analysis
Some of the affected children were positive for IA on their first blood draw, producing left-censored data. We have interval-censored data in that we know only the time of the last negative and first positive autoantibody blood draw, rather than the actual time of conversion to autoantibody positivity. Survival analysis (specifically, accelerated failure-time regression) with the Weibull distribution was used to test the associations between the timing of IA and early childhood illness.
The characteristics of study participants by IA status were examined univariately for potential confounders and covariates, including cohort (FDR vs. GP), HLA risk group, sex of the child, race/ethnicity, birth order, maternal age, any breast-feeding, breast-feeding duration, timing of exposure to foods containing wheat or barley, exposure to daycare, household size, and crowding (number of people in the household dived by the number of rooms in the home). Potential predictors of IA were then examined in survival models. The presence of interactions between early life illnesses and potential effect modifiers, such as infant diet (breast-feeding, introduction of wheat and barley), daycare exposure, and household size and crowding were tested.
The data analysis for this article was generated using SAS software, version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC).
RESULTS
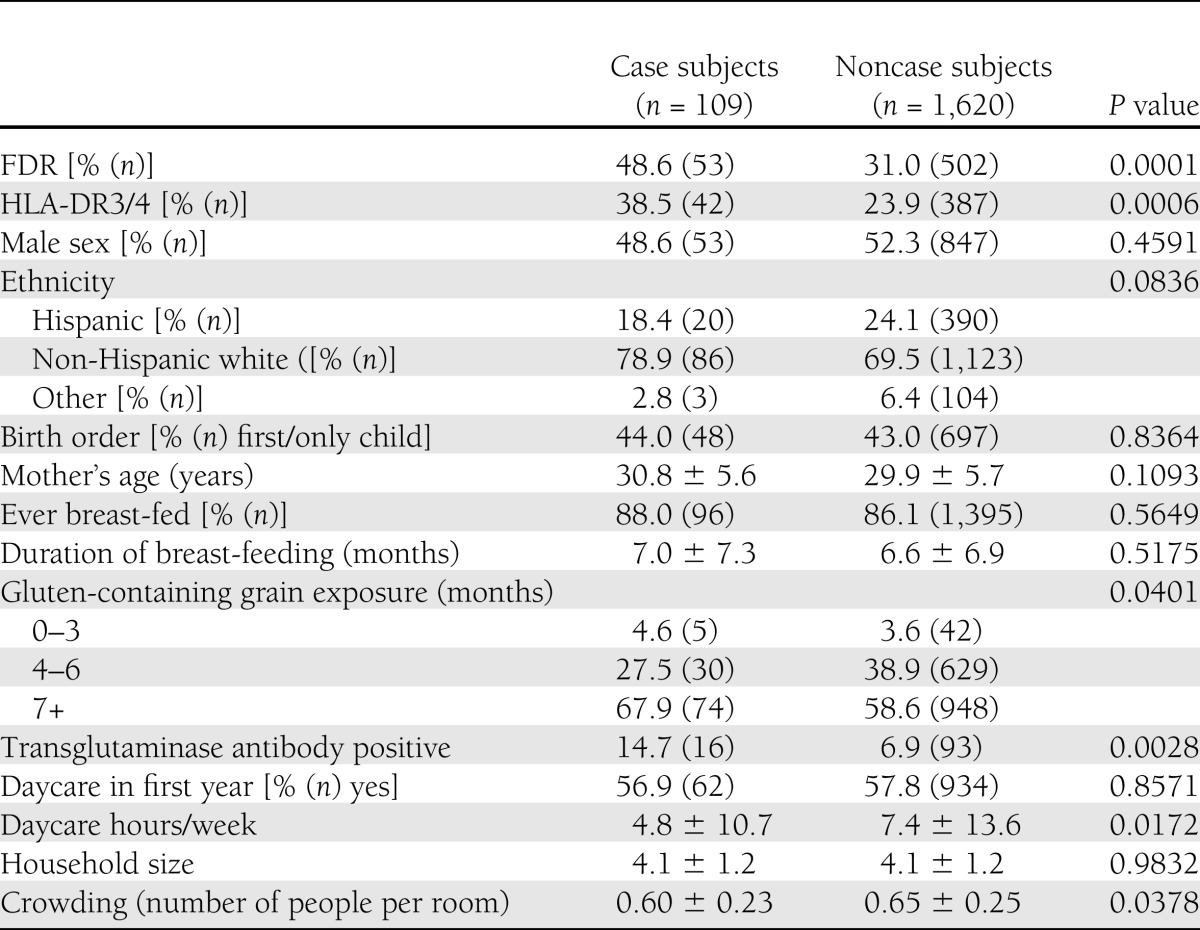
During the course of the study, 124 children developed persistent IA. Of these, we had completed illness questionnaires on 109 (53 from the GP cohort and 56 from the FDR cohort). Characteristics of study participants are shown in Table 1 by IA status. Cases were significantly more likely to have an FDR with type 1 diabetes, transglutaminase antibodies, and a high-risk HLA genotype. In addition, timing of exposure to foods containing gluten (wheat, barley) was significantly different by antibody status, whereas the broader categories of grains were not associated with antibody status. Children with positive antibodies had been exposed to daycare for fewer hours per week and were living in less crowded living situations.
Table 1.
Characteristics of study participants by IA case status
The number of illnesses reported during the first 9 months of life did not vary between those children who did not develop IA versus those who developed IA for GI illnesses (1.65 ± 3.72 vs. 1.10 ± 2.43; P = 0.48), respiratory diseases (0.16 ± 0.53 vs. 0.17 ± 0.47; P = 0.43), fevers (1.34 ± 2.15 vs. 1.16 ± 1.24; P = 0.89), or upper respiratory symptoms (8.20 ± 11.32 vs. 8.08 ± 10.50; P = 0.92).
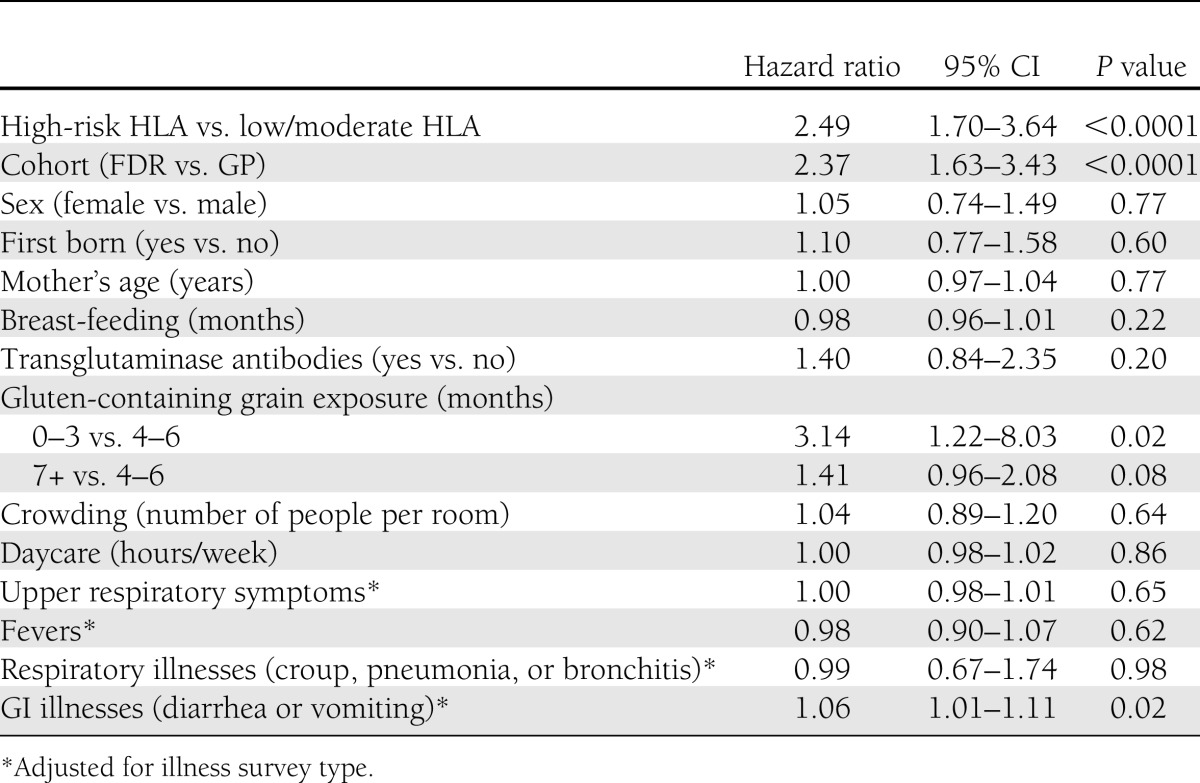
Potential risk factors for IA development were then examined in an accelerated failure-time regression model based on a Weibull distribution (Table 2). Having an FDR with type 1 diabetes and having the high-risk HLA genotype were associated with a higher risk of developing IA. Exposure to wheat or barley either early (0–3 months) or late (7+ months) was associated with an increased risk of developing IA compared with exposure at 4–6 months of age. A greater number of GI illnesses were associated with an increased risk of IA development.
Table 2.
Accelerated failure-time regression models (unadjusted)
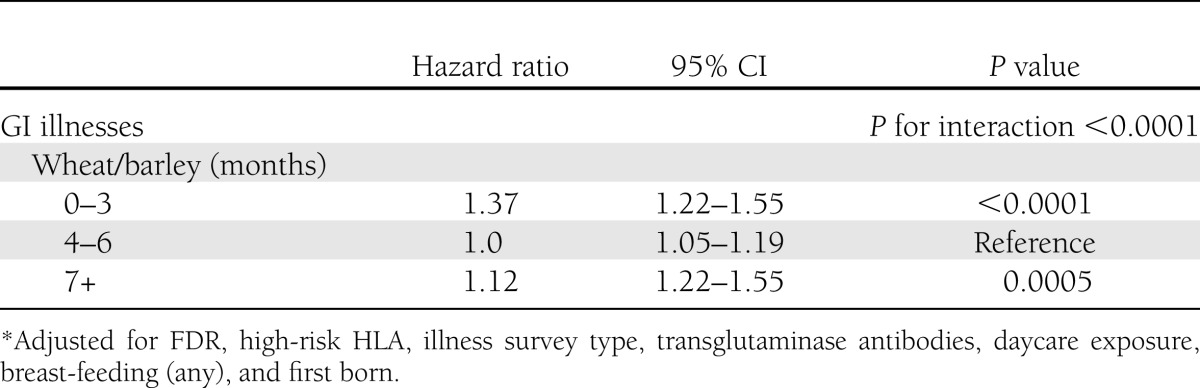
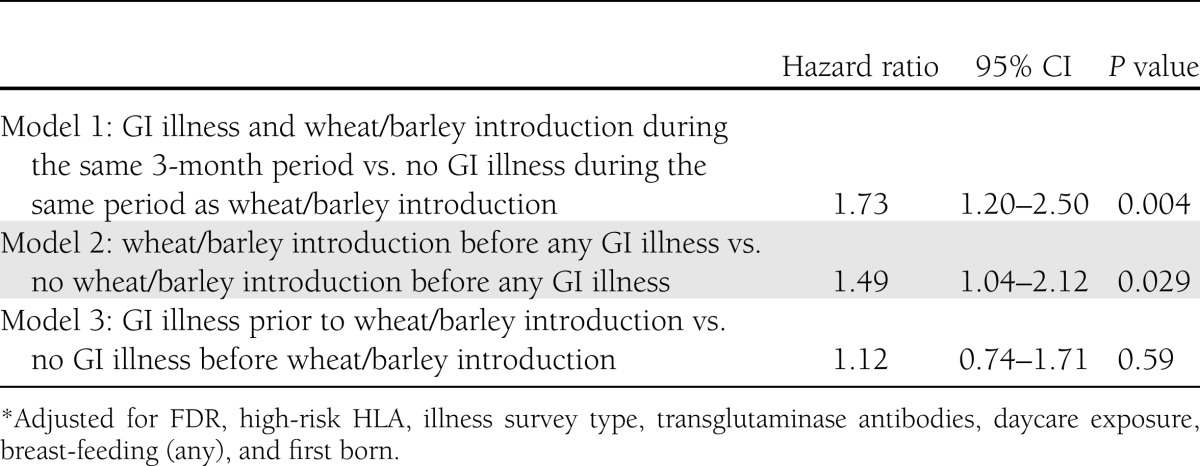
We then tested for interactions between infections and daycare exposure, breast-feeding, and wheat or barley introduction. The only significant interaction (P < 0.05) found was between wheat or barley introduction and GI illnesses, such that the association between GI infections and IA differed by the timing of introduction of foods containing gluten (wheat or barley) (Table 3). Each GI illness was associated with a 37% increased risk of IA development among children exposed to wheat or barley <4 months of age, and a 12% increased risk of IA among children not exposed to wheat or barley until ≥7 months of age. Among children with wheat or barley introduction during the 4–6-month-age period, there was no association of GI illnesses and persistent IA. The relationship between timing of GI illness and timing of introduction of wheat or barley was then further explored in three separate models, as shown in Table 4. When wheat or barley was introduced in the 3-month period preceding a GI illness, or when a GI illness occurred during the same 3-month interval as wheat or barley was introduced, there was a significantly increased risk of antibody positivity compared with those without a GI illness during the time interval when wheat or barley was first introduced (model 1). There was no significant association with IA in children with a GI illness occurring before introduction to wheat or barley compared with children with no GI illness before wheat or barley introduction.
Table 3.
Association of number of GI illnesses with antibody positivity by timing of introduction of gluten (wheat or barley)*
Table 4.
Association of timing of GI illnesses with antibody positivity by timing of introduction of gluten (wheat or barley)*
CONCLUSIONS
In this report, we found that GI illnesses are associated with increased risk of IA among children who are exposed to wheat or barley either early or late in infancy. Although children who attended daycare had significantly more infections during the first 9 months of life, neither the number of hours spent in daycare per week nor the attendance at any daycare was associated with IA.
Childhood infections have been considered as a potential environmental trigger of type 1 diabetes for many years, although the exact nature of this relationship and potential mechanisms are not clear. It has been thought that infectious agents could trigger an immune system response to proteins in the body that appear similar. Alternatively, infection could cause localized inflammation, and chronic infections could therefore lead to a more active immune system, which could be prone to develop autoimmunity. Another theory is that the infectious organisms could activate polyclonal B-cells (24).
A common virus implicated in GI illnesses is rotavirus, which has been shown to have similar protein sequences to T-cell epitopes in the autoantigens GAD and IA-2, perhaps triggering autoimmunity through molecular mimicry. Seroconversion with rotavirus has been demonstrated to increase levels of IA in children with a FDR with type 1 diabetes in the BabyDiab study (25). In addition, animal models have provided evidence that the timing of rotavirus infection may be important. In NOD mice, oral infection with rotavirus accelerated the development of diabetes when insulitis was already present (26). In contrast, infection with rotavirus in young NOD mice without insulitis appears to delay the onset of type 1 diabetes (27).
We have previously reported an association of early and late cereal exposure with the development of IA (5), although this particular exposure variable included oats and rice in addition to wheat and barley. In this report, we found that the association of GI illnesses with IA was only significant among children who were introduced to wheat or barley before 4 months of age or after 6 months of age, and there was a further association among children who had a GI illness during the same 3-month interval as the introduction of wheat or barley or in the 3 months after wheat or barley introduction. Wheat protein antigens can induce an inflammatory response in the gut (28) and so could prime the immune system for a detrimental response to infection with a viral agent. Enteroviruses are the most prevalent viral infectious agents in humans and have been found in pancreatic tissues from patients with type 1 diabetes (29) and in people with islet cell autoantibodies (30). Enteroviruses must enter the epithelial cells of the respiratory or intestinal systems in order to infect the host. Increased gut permeability is found among individuals with IA (31), suggesting that factors leading to a damaged endothelial barrier in the gut, such as gluten exposure, may allow for infection with enteroviruses, which may then damage the pancreatic β-cells. Rotavirus, the most common viral agent causing diarrhea in children, affects gut permeability and intestinal cytokine balance, and so exposure to this virus may have varying effects on immune modulation depending on the concurrent exposure to grain antigens. The data in the current report suggest that exposure to gluten either prior to or during the same 3-month period as a GI illness increases the risk of persistent IA, but it is difficult to tell from these data whether this is attributed to an inflammatory response to gluten antigens or increased gut permeability due to viral illness at the same time as gluten antigens are introduced.
Incidence of type 1 diabetes varies across countries and has been increasing over the past several decades globally. According to a publication from the American Dietetic Association, ∼10% of infants in the U.S. are exposed to grains before 4 months of age, and consumption of grains among those 4–5.9 month of age has decreased from 2002 to 2008 (32), suggesting that grain intake may have been shifted either earlier or later than this time frame over time. In a study of infant feeding in Norway, in which type 1 diabetes incidence is higher than in the U.S., 21% of infants began eating solid foods before 4 months of age (33). Not only the timing of gluten introduction but also the quantity can vary across countries. In countries with high rates of type 1 diabetes such as a Finland and Sweden, there are also varying rates and ages at presentation of celiac disease, with earlier onset and more traditional symptoms in Sweden than in Finland. Ascher et al. (34) reported that wheat protein intake was much higher among infants in Sweden than in Finland at 9 and 12 months of age, perhaps accounting for these differences in celiac disease. More information is needed about how differences in infant feeding across countries and over time may relate to incidence of type 1 diabetes,
Increasing rates of cesarean section may also contribute to the growing incidence of type 1 diabetes, as mode of delivery has been shown to modify the gut flora in infants (35). However, an association between cesarean delivery and risk for developing type 1 diabetes has been inconsistent in various studies (36–39).
In addition to evidence that some childhood infections can increase the risk of IA and type 1 diabetes, it has been noted that the increase in type 1 diabetes incidence has occurred in countries with greater hygiene and less overall rates of infection. This observation has led to the hygiene hypothesis, which postulates that some infections can protect against the development of autoimmune disorders. In the current study, we did not find that an increased number of childhood infections were associated with a lower risk of IA, as proposed by the hygiene hypothesis. Our findings are consistent with those reported by Cardwell et al. (17) in a study of early childhood infections in children with type 1 diabetes and matched controls, in whom common infections in the first year of life were not associated with the later development of type 1 diabetes (17).
There are important limitations to the current study. Childhood infections were reported by the primary caregiver at visits every 3 months during the first 9 months of life. It is possible that some infections were overreported, if they resulted in multiple symptoms (e.g., GI symptoms and upper respiratory symptoms during one infection), or underreported, if they occurred more remotely from the study visit. However, it is unlikely that these errors would have been related to later antibody development, and misclassification because of over- or underreporting of illnesses would bias our results toward the null. Further, it is not possible for us to determine which infectious agent was responsible for each illness, which limits our interpretation of results.
We found that more frequent GI illnesses were associated with increased risk of IA. However, when examined in conjunction with infant diet, we found that the increased risk of IA associated with GI illnesses was only among children first exposed to wheat or barley either early or late, particularly if a GI illness occurred during the same interval as the cereal introduction or just after. As a result, it appears that pathogens affecting the GI system may increase the risk of IA in the presence of existing inflammation induced by diet.
Acknowledgments
This study was funded by grants R01-DK32493 and R01-DK49654 from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; by Diabetes and Endocrinology Research Center, National Institutes of Health Grant P30-DK-57516; and by the American Diabetes Association Junior Faculty Award 1-10-JF-50 (J.K.S.-B.).
No potential conflicts of interest relevant to this article were reported.
J.K.S.-B. wrote the manuscript and analyzed data. J.S. researched data, wrote the manuscript, and reviewed and edited the manuscript. F.D. and A.E.B. reviewed and edited the manuscript and analyzed data. K.B., J.M.N., and M.R. researched data, reviewed and edited the manuscript, and contributed to discussion. J.K.S.-B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
References
- 1.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007;30:503–509 [DOI] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 [DOI] [PubMed] [Google Scholar]
- 4.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360:1646–1654 [DOI] [PubMed] [Google Scholar]
- 5.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290:1713–1720 [DOI] [PubMed] [Google Scholar]
- 6.Sipetić S, Vlajinac H, Kocev N, Bjekić M, Sajic S. Early infant diet and risk of type 1 diabetes mellitus in Belgrade children. Nutrition 2005;21:474–479 [DOI] [PubMed] [Google Scholar]
- 7.Pflüger M, Winkler C, Hummel S, Ziegler AG. Early infant diet in children at high risk for type 1 diabetes. Horm Metab Res 2010;42:143–148 [DOI] [PubMed] [Google Scholar]
- 8.Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr 2011;94(Suppl.):1821S–1823S [DOI] [PubMed] [Google Scholar]
- 9.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003;290:1721–1728 [DOI] [PubMed] [Google Scholar]
- 10.Sipetic S, Vlajinac H, Marinkovi J, et al. Stressful life events and psychological dysfunctions before the onset of type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2007;20:527–534 [DOI] [PubMed] [Google Scholar]
- 11.Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Rev Endocr Metab Disord 2006;7:149–162 [DOI] [PubMed] [Google Scholar]
- 12.Kolb H, Elliott RB. Increasing incidence of IDDM a consequence of improved hygiene? Diabetologia 1994;37:729. [DOI] [PubMed] [Google Scholar]
- 13.Cooke A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of type 1 diabetes? Immunology 2009;126:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–485 [DOI] [PubMed] [Google Scholar]
- 15.Schwimmbeck PL, Dyrberg T, Oldstone MB. Abrogation of diabetes in BB rats by acute virus infection. Association of viral-lymphocyte interactions. J Immunol 1988;140:3394–3400 [PubMed] [Google Scholar]
- 16.Gibbon C, Smith T, Egger P, Betts P, Phillips D. Early infection and subsequent insulin dependent diabetes. Arch Dis Child 1997;77:384–385 [DOI] [PubMed] [Google Scholar]
- 17.Cardwell CR, Carson DJ, Patterson CC. No association between routinely recorded infections in early life and subsequent risk of childhood-onset Type 1 diabetes: a matched case-control study using the UK General Practice Research Database. Diabet Med 2008;25:261–267 [DOI] [PubMed] [Google Scholar]
- 18.Stene LC, Barriga K, Norris JM, et al. Symptoms of common maternal infections in pregnancy and risk of islet autoimmunity in early childhood. Diabetes Care 2003;26:3136–3141 [DOI] [PubMed] [Google Scholar]
- 19.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 20.LaGasse J, Jelinek L, Sexson S, et al. An islet-cell protein tyrosine phosphatase is a likely precursor to the 37-kDa autoantigen in type 1 diabetes: human and macaque sequences, tissue distribution, unique and shared epitopes, and predictive autoantibodies. Mol Med 1997;3:163–173 [PMC free article] [PubMed] [Google Scholar]
- 21.Babaya N, Yu L, Miao D, et al. Comparison of insulin autoantibody: polyethylene glycol and micro-IAA 1-day and 7-day assays. Diabetes Metab Res Rev 2009;25:665–670 [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Eisenbarth G, Bonifacio E, Thomas J, Atkinson M, Wasserfall C. The second murine autoantibody workshop: remarkable interlaboratory concordance for radiobinding assays to identify insulin autoantibodies in nonobese diabetic mice. Ann N Y Acad Sci 2003;1005:1–12 [DOI] [PubMed] [Google Scholar]
- 23.Kleinman RE. American Academy of Pediatrics recommendations for complementary feeding. Pediatrics 2000;106:1274. [PubMed] [Google Scholar]
- 24.Goldberg E, Krause I. Infection and type 1 diabetes mellitus - a two edged sword? Autoimmun Rev 2009;8:682–686 [DOI] [PubMed] [Google Scholar]
- 25.Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 2000;49:1319–1324 [DOI] [PubMed] [Google Scholar]
- 26.Graham KL, Sanders N, Tan Y, Allison J, Kay TW, Coulson BS. Rotavirus infection accelerates type 1 diabetes in mice with established insulitis. J Virol 2008;82:6139–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham KL, O’Donnell JA, Tan Y, et al. Rotavirus infection of infant and young adult nonobese diabetic mice involves extraintestinal spread and delays diabetes onset. J Virol 2007;81:6446–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakir H, Lefebvre DE, Wang H, Caraher E, Scott FW. Wheat protein-induced proinflammatory T helper 1 bias in mesenteric lymph nodes of young diabetes-prone rats. Diabetologia 2005;48:1576–1584 [DOI] [PubMed] [Google Scholar]
- 29.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 30.Williams CH, Oikarinen S, Tauriainen S, Salminen K, Hyöty H, Stanway G. Molecular analysis of an echovirus 3 strain isolated from an individual concurrently with appearance of islet cell and IA-2 autoantibodies. J Clin Microbiol 2006;44:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006;49:2824–2827 [DOI] [PubMed] [Google Scholar]
- 32.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc 2010;110(Suppl.):S38–S51 [DOI] [PubMed] [Google Scholar]
- 33.Lande B, Andersen LF, Baerug A, et al. Infant feeding practices and associated factors in the first six months of life: the Norwegian infant nutrition survey. Acta Paediatr 2003;92:152–161 [DOI] [PubMed] [Google Scholar]
- 34.Ascher H, Holm K, Kristiansson B, Mäki M. Different features of coeliac disease in two neighbouring countries. Arch Dis Child 1993;69:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 1999;28:19–25 [DOI] [PubMed] [Google Scholar]
- 36.Stene LC, Magnus P, Lie RT, Søvik O, Joner G, Norwegian Childhood Diabetes Study Group No association between preeclampsia or cesarean section and incidence of type 1 diabetes among children: a large, population-based cohort study. Pediatr Res 2003;54:487–490 [DOI] [PubMed] [Google Scholar]
- 37.McKinney PA, Parslow R, Gurney K, Law G, Bodansky HJ, Williams DR. Antenatal risk factors for childhood diabetes mellitus; a case-control study of medical record data in Yorkshire, UK. Diabetologia 1997;40:933–939 [DOI] [PubMed] [Google Scholar]
- 38.Patterson CC, Carson DJ, Hadden DR, Waugh NR, Cole SK. A case-control investigation of perinatal risk factors for childhood IDDM in Northern Ireland and Scotland. Diabetes Care 1994;17:376–381 [DOI] [PubMed] [Google Scholar]
- 39.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51:726–735 [DOI] [PubMed] [Google Scholar]