The concept that type 2 diabetes mellitus (T2DM) can be reversed with an intestinal operation is counterintuitive. How could our costliest disease be forced into full, durable, and safe remission with the bypass of a few inches of intestine?
Counterintuitive or not, it’s true. Accordingly, we take notice when Sjöström and colleagues (1) in the Swedish Obese Subjects (SOS) study, the longest and most complete bariatric surgery outcome study in the world, document in this issue that bariatric surgery reduces the incidence of heart attacks. The SOS is a prospective, nonrandomized, controlled interventional trial on the effect of bariatric surgery on mortality and morbidity compared with conventional treatment that enrolled 4,047 obese individuals from 1 September 1987 to 31 January 2001. Of these, 2,010 underwent bariatric surgery, and a contemporary matched group of 2,037 did not. The current report compared the 345 diabetic patients who underwent bariatric surgery with the 262 who did not. The authors found that “bariatric surgery was associated with a reduced myocardial infarction incidence” (38/345 [11.0%] in the surgery vs. control group 43/262 [16.4%] [P = 0.017]). The effect was stronger in individuals with higher serum cholesterol and triglycerides at baseline. Not surprising, since the bariatric surgery was associated with significant decreases in body weight, blood glucose, serum triglycerides, systolic and diastolic blood pressure, and an increase in HDL-cholesterol.
Others have reported similar benefits of bariatric surgery on cardiovascular disease and mortality. Johnson et al. (2) mined the data in the South Carolina UB92 Inpatient Hospitalization Database and Death Records and concluded that in a cohort study of 349 bariatric surgical patients and 903 control subjects that “adjusting for age, comorbidities, and event history, the relative risk of mortality was reduced by 40 per cent in bariatric patients compared with controls.” Similarly, Batsis et al. (3), calculated the Framingham and the Prospective Cardiovascular Munster cardiovascular risk scores in 197 patients who underwent the Roux-en-Y gastric bypass (RYGB) versus a matched cohort of 163 individuals who did not. With both scoring methods, cardiovascular relative risk was reduced from 79 to 18% with the Framingham instrument and from 62 to 8% to with the Prospective Cardiovascular Munster scores. In our study (4) of 232 severely obese diabetic patients of whom 154 underwent bariatric surgery versus 78 individuals who were also scheduled for but did not undergo the operations mainly because of failure to obtain insurance coverage, the mortality was 78% less in the operated group. This report from the SOS, in addition to their previous reports, as well as the extensive publications of other clinical series throughout the world, should now be ample documentation that bariatric surgery is effective, efficient, and safe.
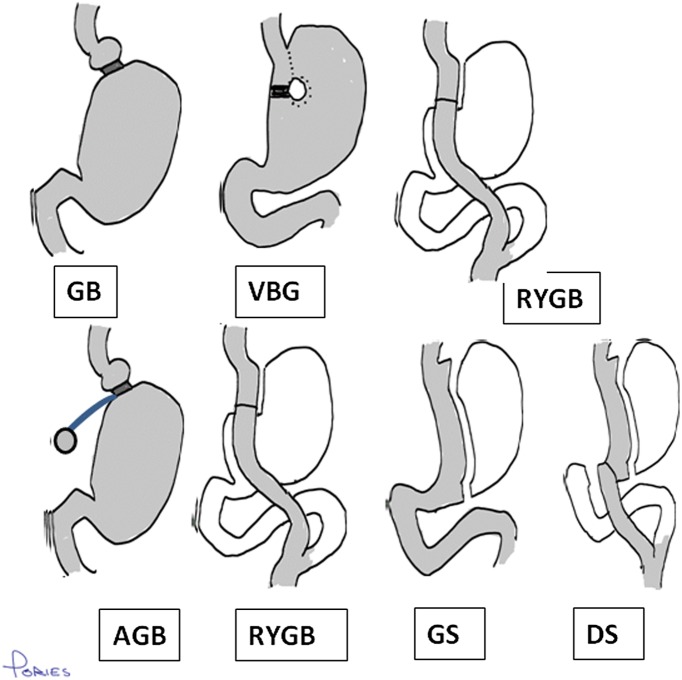
Unfortunately, the relevance of the SOS series is increasingly limited because the operations used in the study no longer reflect the practices of today. The vertical banded gastroplasty performed in 227 of the 345 (65.8%) patients is no longer done. Their version of the vertical banding performed in 61 (17.7%) patients was not adjustable, and the 57 gastric bypasses (RYGB) (16.5%) had alimentary limbs that were shorter (60 cm) than those used currently (100–150 cm). Today’s four accepted operations, the adjustable gastric band, the gastric sleeve, the RYGB, and the biliopancreatic bypass with a duodenal switch are far more effective in controlling type 2 diabetes than these older procedures. For example, our recent meta-analysis (5) involving 621 studies published in the English language involving 135,246 patients found diabetes remission rates of 56.7% for the vertical banded gastroplasty and 95.1% for the biliopancreatic bypass with duodenal switch. In short, the reduction in myocardial infarctions in the SOS series was achieved with operations that were less effective in the treatment of T2DM than those used today. For clarification of this surgical jargon, Fig. 1 provides an overview of these operations.
Figure 1.
Diagrams of bariatric operations. Shading indicates contact of food with the gut wall. The top line indicates the three operations in the SOS study: gastric banding (GB), vertical banded gastroplasty (VBG), and RYGB. The lower line illustrates the four commonly performed procedures today: adjustable gastric banding (AGB), RYGB, gastric sleeve (GS), and biliopancreatic bypass with duodenal switch (DS). The limb lengths are schematic; current alimentary limb lengths are 100–150 cm in the RYGB, whereas the biliopancreatic bypass with duodenal switch excludes most of the small bowel to a short common channel 100 cm proximal to the ileocecal junction.
The second problem with the SOS study is that the operations and the perioperative care were not standardized in the participating 25 surgical departments—frankly, a concern that has still not been addressed in the most bariatric surgical series published today. We may refer to the “gastric bypass operation” as a single defined procedure, but these operations actually differ significantly across the U.S. in terms of the surgical approaches: 1) open versus laparoscopic; 2) sizes of the gastric pouches; 3) direction of the pouches, i.e., horizontal versus vertical; 4) the diameters of the gastroenterostomy; 5) construction of the gastroenterostomy, i.e., stapled versus hand sewn; 6) routing of the jejunal loops (antecolic vs. retrocolic); 7) length of the biliopancreatic limb; 8) length of the alimentary limbs; 9) sizes of the jejuno-jejunostomy; 10) use of drains; and 11) use of limiting gastric bands—if you do the math, it turns out there are over 1,000 variations. In addition, and of special relevance to metabolic studies, protocols generally do not standardize perioperative care, including specification of anesthetic agents, antibiotics, and other drugs. We really need to address this serious issue in the design of future studies.
Another finding in the SOS study that deserves comment is that “baseline fasting insulin levels rather than BMI predicted the surgery treatment benefit on cardiovascular event.” The failure of the BMI (kg/m2) as a predictive tool is not surprising. The BMI, a simple measure of height over weight that is easily captured in a clinical visit, may be a useful tool for epidemiologic studies, but it is not at all a reliable measure of adiposity. It fails to allow for differences in sex, race, age, fitness, and fat distribution (6). This is a serious issue because access to bariatric surgery is denied by insurance carriers unless the patient’s BMI is ≥40 or ≥35 in those with serious comorbidities. This faulty measure discriminates primarily against those who need it most: the poor, females, Asians, and African Americans. The continued use of the BMI as a cruel gate to deny patients access to the surgery is ever harder to explain.
Their finding that fasting insulin levels can predict outcomes is also of interest. This fits with the observations that 1) basal levels of insulin increase with the progression of T2DM and 2) gastric bypass produces simultaneous correction of hyperglycemia and hyperinsulinemia (also counterintuitive) in a matter of days (7,8). It would be a major boon if this simple test proved to be a reliable predictor of bariatric outcomes.
Our Swedish colleagues have made major contributions to our understanding of bariatric surgery. However, we need to move on. The evidence, counterintuitive or not, is in. Durable and full remission of T2DM is now achieved daily in the U.S. in over 450 certified Centers of Excellence. The task now is to use the opportunities offered by bariatric surgery to explore the mechanisms that produce T2DM. We have some tantalizing clues from recent studies of the microbiome, gut signaling in response to food, incretin function, insulin action, mitochondrial responses, and control of energy metabolism. The more rapidly we pursue these questions, the greater our chances for finding medications that can rival the surgical results.
So why is S.O.S.—a tragic, ageless mnemonic that screams “save our souls”—in the title of this commentary? It is there to emphasize that less than 1% of those who could benefit from bariatric surgery have access to this remarkably effective, life-saving treatment. Our patients deserve better. S.O.S!
Acknowledgments
W.J.P. receives grant funding from the National Institutes of Health.
W.J.P. also receives grant funding from Johnson & Johnson, HERSA, and Golden Leaf. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See Romeo et al., p. 2613
References
- 1.Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RJ, Johnson BL, Blackhurst DW, et al. Bariatric surgery is associated with a reduced risk of mortality in morbidly obese patients with a history of major cardiovascular events. Am Surg 2012;78:685–692 [PubMed] [Google Scholar]
- 3.Batsis JA, Sarr MG, Collazo-Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol 2008;102:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald KG, Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997;1:213–220; discussion 220 [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256, e5 [DOI] [PubMed] [Google Scholar]
- 6.Pories WJ, Dohm LG, Mansfield CJ. Beyond the BMI: the search for better guidelines for bariatric surgery. Obesity (Silver Spring) 2010;18:865–871 [DOI] [PubMed] [Google Scholar]
- 7.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care 2012;35:2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]