Figure 3.
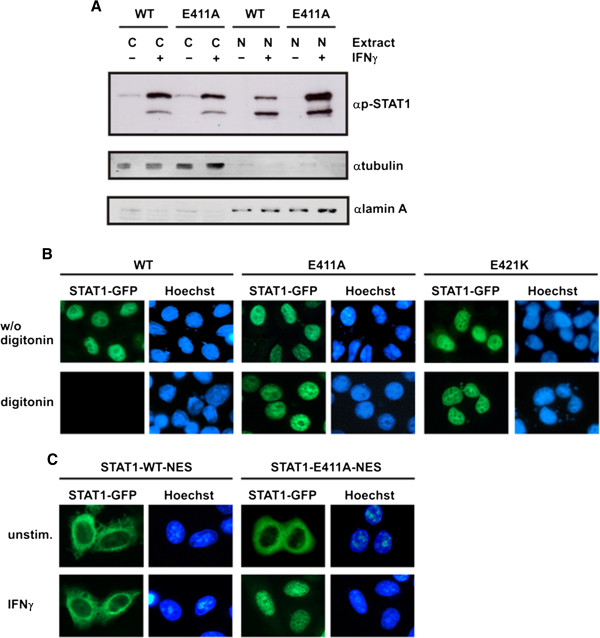
Mutation of E411 results in diminished nuclear export of STAT1. (A) Altered nucleocytoplasmic distribution of the E411A mutant. HeLa cells expressing GFP-tagged STAT1 variants were either unstimulated (−) or stimulated with IFNγ (+), as indicated. The levels of phosphorylated STAT1 were probed separately in cytosolic and nuclear extracts by means of Western blotting using a phospho-tyrosine-specific STAT1 antibody. Similar amounts of endogenous phospho-STAT1 in both transfections were loaded onto the gel (lower band). The concentration of phosphorylated STAT1-E411A-GFP (upper band) in nuclear extracts (N) exceeded that of the wild-type, whereas, conversely, in cytosolic extracts (C) there were lower amounts as compared to the wild-type protein. The purity of cytoplasmic/nuclear extracts was assessed by simultaneous incubation of the blot membrane with anti-ß-tubulin and anti-lamin A antibodies followed by secondary species-specific IRDye 680LT and 800CW antibodies. (B) Reduced nuclear export kinetics of STAT1-E411A and -E421K. HeLa cells expressing fusions of green fluorescent protein with either wild-type or mutant STAT1 were prestimulated for 45 min with IFNγ to induce nuclear accumulation (top panel) and then treated for 6 min in the presence of 50 μg/ml digitonin in transport buffer (bottom panel). Fluorescence micrographs of formaldehyde-fixed cells are shown, demonstrating the amount of nuclear STAT1-GFP and the localization of the corresponding Hoechst-stained nuclei. (C) The E411A mutation restores defective nuclear accumulation of the STAT1-NES-GFP construct. HeLa cells were transfected with pSTAT1-NES-GFP, which coded for a transferable nuclear export signal (NES) situated between the cDNAs for full-length STAT1 and GFP. Cells expressing wild-type STAT1-NES-GFP or the respective E411A variant thereof were either left untreated or stimulated with IFNγ.
