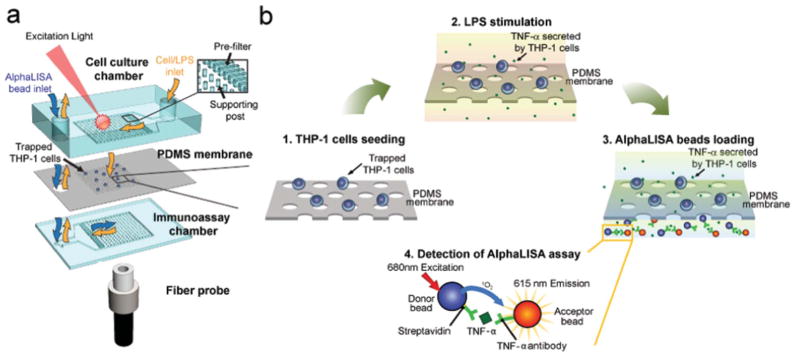
Fig. 1.
Functional immunophenotyping using the MIPA device (a) Schematic of the multi-layered MIPA device consisting of a cell culture chamber, a PDMS microfiltration membrane (PMM), and an immunoassay chamber. The size of both the cell culture and immunoassay chambers is 3.7 mm (L: length) × 3 mm (W: width) × 100 μm (H: height). The inset shows the pre-filter structure (300 μm L × 50 μm W × 100 μm H) to block particles larger than 50 μm in diameter (e.g. aggregated cells) and the supporting posts to prevent deformation of the PMM. The supporting post diameter is 50 μm with a center-to-center distance of 200 μm. A fiber probe was attached underneath the immunoassay chamber to collect AlphaLISA emission signal. (b) Schematic showing the immunophenotyping assay protocol used in this study: (1) Isolation and enrichment of THP-1 cells on the PMM; (2) LPS-stimulation of cells; (3) Loading and incubation of AlphaLISA beads in the immunoassay chamber; (4) TNF-α detection using the AlphaLISA assay, in which the streptavidin-coated donor (blue) and acceptor beads (orange) are both conjugated with TNF-α antibodies. The beads are brought into close proximity (<200 nm) through binding simultaneously to TNF-α. Using 680 nm laser for excitation, the singlet oxygen released by the donor bead diffuses to the nearby acceptor bead and triggers it to emit 615 nm fluorescent light.