Abstract
Purpose
The aim of this study was to present our technique to implant unicompartmental knee arthroplasty (UKA) using navigation and to give our first results regarding the accuracy of the device.
Methods
A total of 33 patients with medial femorotibial osteoarthritis (31) or avascular necrosis (2) were included in this study. The mean preoperative hip-knee-ankle (HKA) angle was 172.7 ± 2.2° (range 167–177°) and the preoperative planning aimed to reach an HKA angle between 175 and 179° (177 ± 2°), a tibial varus at 3 ± 1°, which means a tibial mechanical angle (TMA) close to 87 ± 1°, and posterior tibial slope at 3 ± 2°. In all cases, we used the OrthoPilot® device with dedicated software allowing us to navigate only the tibial plateau.
Results
The preoperative plan was reached in 93.9 % of cases for HKA angle, 84.8 % for TMA and 100 % for the posterior slope.
Conclusions
Unicompartmental knee navigation is reliable. The navigation of only the tibial bone cut is a reasonable option as has been shown in this study. Its role is invaluable in the positioning of mobile-bearing UKA, where the risk of overcorrection should not be underestimated.
Introduction
Over the last ten years, less invasive and minimally invasive (MIS) surgery has re-popularised unicompartmental knee arthroplasty (UKA) [1]. UKA is challenging, as much for its indication as for its surgical technique, and it had not seen any considerable improvements for decades. At the beginning of the 1970s, Marmor [2] pioneered a technique for UKA (tibial plateau and femoral condyle replacement) with rudimentary mechanical instrumentation, during which surgeons “eyeballed” the position of the prosthesis, a method which often resulted in obvious inaccuracies. At the beginning of the 1980s, Cartier and Cheaib [3] introduced more modern mechanical tibial jigs, in order to improve bone cut reproducibility, as well as trial implants which allowed for more reliable intraoperative testing. At the beginning of the 1990s, due to the difficulties related to the positioning of the femoral implant, intramedullary alignment instrumentation emerged, which finally transformed relatively less invasive surgery into a more invasive technique, similar to the one used in total knee arthroplasty (TKA), which was not the original goal of UKA. However, the UKA technique remains challenging due to the lack of intraoperative control, which relies mostly on the surgeon’s experience. Surgical experience in UKA remains difficult to acquire looking at the limited number of UKA carried out in orthopaedic surgical practice.
It has been shown that the survivorship of UKA is mainly related to leg alignment [1, 4–6]. Leg alignment hypercorrection after UKA leads to early wear on the opposite side of the knee joint, and conversely hypocorrection can lead to premature wear of the implant, or worse, tibial implant loosening. The premature failure of a tibial implant of UKA also depends on the sagittal position (slope) and, most importantly, on the residual laxity (so-called safe laxity as described by Cartier and Cheaib [3]). Indeed, too much laxity increases unilateral knee instability, which is very detrimental in mobile-bearing UKA (potential meniscal bearing dislocation [7, 8]), and a lack of laxity increases the risk of overcorrection. Conventional UKA surgical techniques do not allow for impartial and reproducible assessment of the safe laxity as there is no control of the femorotibial mechanical angle (FMTA) other than the surgeon’s visual assessment.
Computer-assisted surgery for TKA began at the end of the 1990s [9, 10] and it soon became apparent that it was a remarkable tool on the one hand in fulfilling preoperative planning of the FMTA, and on the other hand in measuring pre- and post-implant mediolateral laxity [11]. Computer-assisted navigation systems have since been used in the positioning of UKA and the initial results were very encouraging [12–18]. UKA navigation can be done in two ways: either by navigating only the tibial cut while the femoral cuts are performed using conventional instrumentation based on the tibial cut or by navigating both the tibial and the femoral cuts. The aim of this study was to evaluate our outcome of 33 medial UKA using surgical navigation techniques navigating only the tibial bone cut.
Materials and methods
The navigation device
A non-image-based/computed tomography (CT)-free navigation system was used (OrthoPilot®, B. Braun/Aesculap, Tuttlingen, Germany). This system is based on kinematic and anatomical registration during which hip, knee and ankle (HKA) kinematic centres are acquired as well as anatomical landmarks. The navigation system is set up with a PC computer, an infrared Polaris camera (Northern Digital Inc., Toronto, ON, Canada) and a double foot pedal. Surgical steps are defined by UKA software and the surgeon progresses through each step using the double foot pedal control displaying the sequence on the monitor screen.
Fixed 3D arrays were placed both in the femur and in the tibia using bicortical screws, and passive markers were used instead of active cable markers which are more cumbersome.
Pre-calibrated mechanical jigs were tracked and allowed the surgeon to navigate the tibial cut for height, varus/valgus and slope. Moreover, the system can be used to navigate the femoral cut although it was not used in this series.
Surgical technique
Preoperative planning included standard X-ray [anteroposterior (AP) and lateral standing, AP in so-called schuss position or Rosenberg view and a skyline view] and a long-leg film (HKA) to measure leg alignment and proximal tibial varus. Sagittal tibial slope was measured on the standard lateral X-ray.
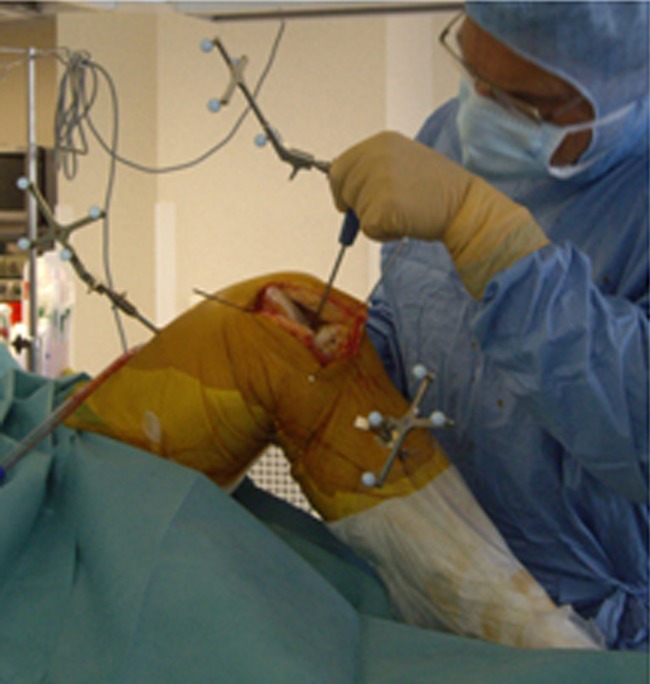
The patient was in a supine position, using a tourniquet, and the computer navigation system was placed 1.8–2.2 m away from the knee on the patient’s opposite side, closer to the patient’s head. First, the 3D arrays were fixed through percutaneous incisions. The femoral tracker was situated about 10 cm above the patella with 45° obliquity from inside to outside with respect to the knee’s coronal plane. The tibial tracker was fixed 10 cm below the femorotibial joint line parallel to the coronal plane in order to avoid any disruption during surgical manipulation (Fig. 1).
Fig. 1.

Percutaneous fixation of the 3D arrays (proximal part of the tibial and distal part of the femur)
A 7- to 9-cm medial parapatellar skin incision was performed and its length depended on the patient’s body mass index (BMI) and soft tissue elasticity. Then a sub-vastus or mid-vastus intra-articular approach was used to access the knee.
The next step was the registration of the hip kinematic centre using hip circumduction movement, then the knee kinematic centre using flexion extension and rotation axis of the knee in 90° flexion and finally the ankle kinematic centre was acquired through its flexion extension movement. The second step of registration involved data collection from the knee: middle of the intercondylar notch, the middle of the tibial spines (Fig. 2), the middle of the medial tibial plateau, the more distal point of the femoral condyle, the posterior condyle, the medial epicondyle, the lateral epicondyle (percutaneous landmark) and finally the medial, lateral malleoli and the middle of the ankle joint.
Fig. 2.
Palpation of the middle of the tibial spines
At the end of the registration, the FMTA was displayed on the monitor screen and the surgeon then started to navigate the tibial bone cut resection. Before proceeding to tibial cutting guide navigation, the correlation between FMTA displayed on the computer monitor screen and the measured preoperative HKA X-ray angle was checked and the reducibility of the leg deformity assessed. If the deformity was hypercorrectable (too much valgus), mobile UKA was contraindicated due to the risk of hypercorrection. Indeed, if the leg stayed in varus, the medial collateral ligament (MCL) will remain slack and the mobile-bearing surface was at risk of dislocation. A bigger polyethylene spacer would fill the gap but this would then increase load stress on the other side of the joint.
The tracked tibial cutting jig was then positioned in front of the medial tibial plateau; navigated and fixed using three to four threaded pins (Fig. 3). Using the graphic user interface (Fig. 4), the tibial jig was placed between 2 and 3° varus and between 3 and 5° slope with a bone resection ranging between four and eight millimetres (never more than eight millimetres in order to avoid any subsidence of the tibial plateau implant related to weak cancellous bone). However, the less varus the more bone resection and vice versa according to the indications for UKA [8]. Once these three parameters (coronal, sagittal and height) were satisfactory the tibial cut was performed using an oscillating saw for the horizontal cut and a reciprocating saw for the vertical cut.
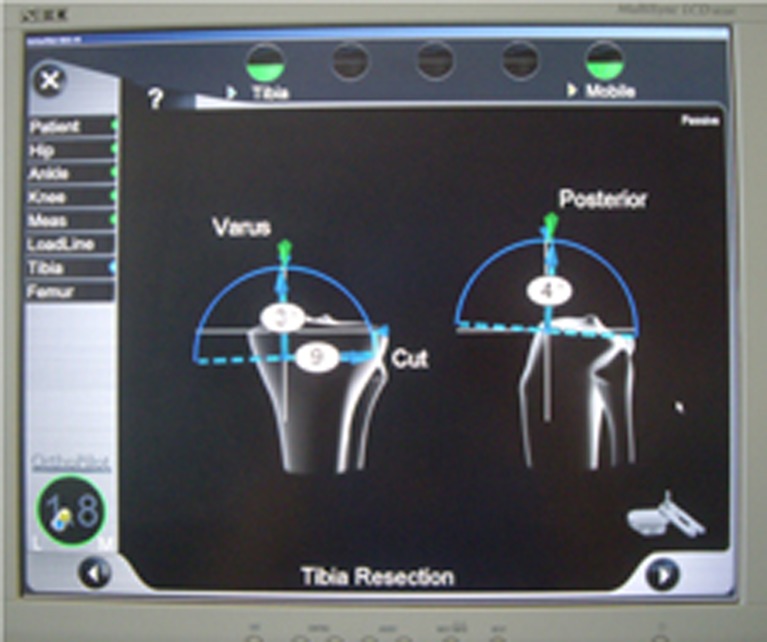
Fig. 3.
Snapshot of the computer screen showing tibial cut angle selection: 3° of varus, 4° of posterior slope and 9 mm of resection height
Fig. 4.
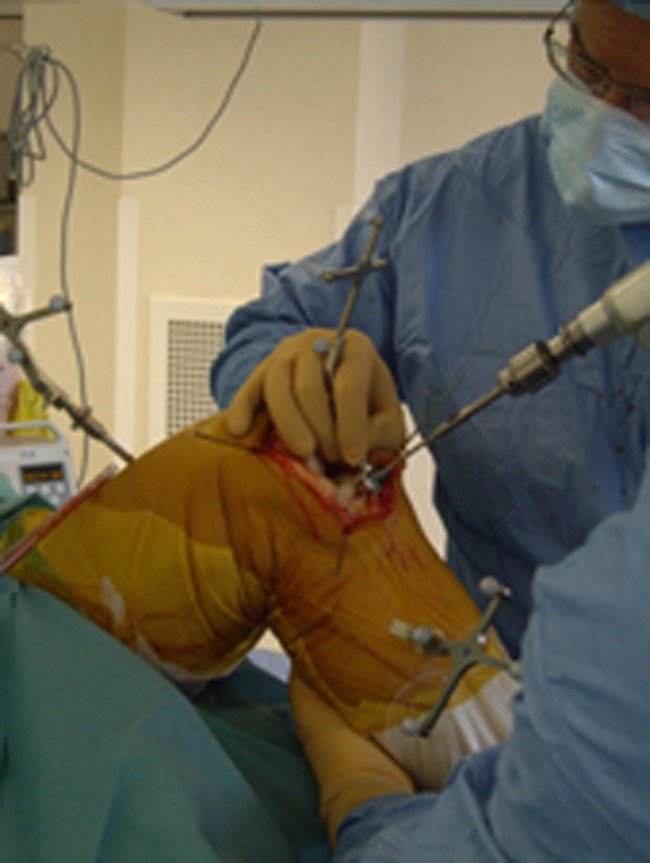
Fixation of the tibial cutting guide equipped with the 3D array
At this stage, the navigation was finished and the femoral cut was performed using a tibial spacer placed between the resected tibial plateau bony surface and the distal medial femoral condyle with the knee in extension. This spacer enabled a slight extension lag between five and ten degrees to be achieved, but never any hyperextension in order to avoid any unwarranted anterior medial condyle resection causing patellofemoral impingement.
The distal femoral jig was slotted onto the tibial spacer until it reached the femur, ensuring that the tibial plateau fitted perfectly well onto the resected bony surface (Fig. 5). Two threaded pins were used to stabilise the femoral jig and the distal femoral cut was performed using an oscillating saw. Then the knee was flexed to 90°, and by using several templates the best-size fit was chosen for the femoral condyle. Posterior and chamfer cuts were performed using the appropriate template. The tibial and femoral trial implants were tested. The surgeon verified leg alignment which should be within 177 ± 2° that is a slight hypocorrection. When using a mobile-bearing tibial plateau, a safe laxity is tolerated at around 1°. If the safe laxity is more than 2°, the choice will then be to use a fixed-bearing tibial plateau. According to the usual UKA technique recommendations, preoperative varus (FMTA) less or equal to ten degrees led exceptionally to hyporeduction greater than five degrees correction, which might need the MCL “pie-crusting” technique in order to lengthen it or to convert from UKA to TKA. Once satisfactory alignment and position were obtained, the final implants were cemented and post-implant FMTA was checked and recorded before closing.
Fig. 5.
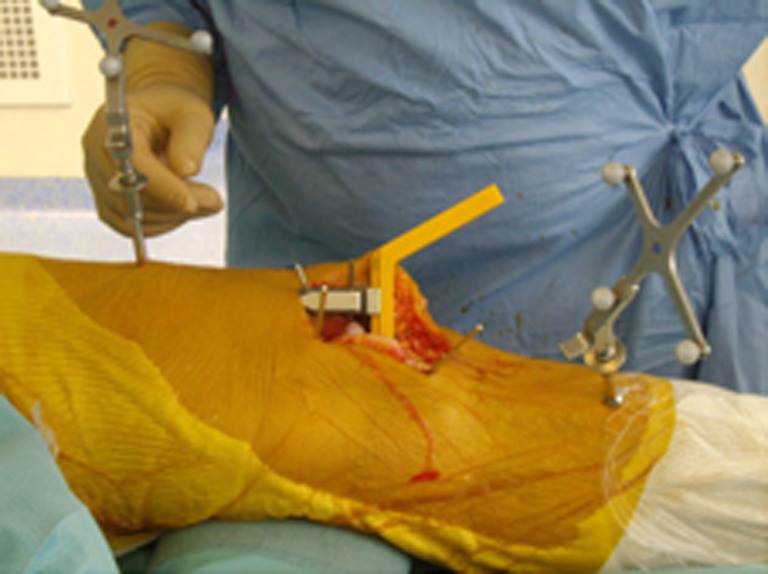
Insertion of the distal femoral cutting guide lying between the femur and the tibial spacer
Patients
A total of 33 patients were included in this study: 15 women and 18 men with a mean age of 70.3 ± 7.7 years (range 59–84). There were two cases of osteonecrosis of the medial condyle and 31 of osteoarthritis including nine stage 2, 17 stage 3 and five stage 4. Preoperative HKA angle was 172.7 ± 2.2° (range 167–177°). The KAPS® UKA (X.NOV, Belfort, France) was implanted in all cases, using either mobile- (31 cases) or fixed-bearing surface (two cases).
Preoperative planning aimed to reach an HKA angle between 175 and 179° (177 ± 2°), a tibial varus at 3 ± 1°, which means a tibial mechanical angle close to 87 ± 1°, and posterior tibial slope at 3 ± 2°.
Assessment review
The patients were all reviewed after three months with AP X-rays and HKA long-leg films using the same protocol as used preoperatively. An independent reviewer measured all X-rays.
Results
Post-implant mean HKA angle was 177.4 ± 1.7° (range 173–182°). The tibial mechanical angle (TMA) average was 86.6 ± 1.2° (range 85–90°) and posterior tibial slope average 3.6 ± 1° (2–5°).
The preoperative plan was achieved in 93.9 % of cases for HKA angle, 84.8 % for TMA and 100 % for the posterior slope.
Discussion
As in TKA, UKA implant position has been proven to be a major factor in the long-term outcome [1, 4–6]. Expected alignment after UKA is still controversial [19]. However, it is generally accepted that malalignment leads to early polyethylene wear and implant loosening [20, 21]. Therefore, it seems reasonable to favour surgical techniques improving accuracy and reproducibility in UKA.
As in TKA, conventional instrumentation which uses visual alignment jigs or intramedullary rods does not ensure optimal UKA implant position [19]. Navigation has been used to improve the reproducibility of the surgical technique, and it has been extensively demonstrated to be efficient [14, 16]. Since then, several studies have confirmed the effectiveness of navigation improving UKA implantation [15, 17, 22], whereas one article did not show any improvement [23].
In this series, the use of navigation by only navigating the tibial bone cut showed an outcome comparable to other series in the literature where authors did navigate both tibial and femoral implants.
We used the so-called dependent cut instrumentation, which is similar to the KAPS® prosthesis conventional technique. Nonetheless, in this technique the tibial cut is made using extra-articular jig instrumentation; only the tibial cutting guide is navigated, not the femur, which reduces the number of instruments used in the field of surgery. Using this method may reduce the operative time with respect to other methods using navigation for both tibial and femoral jigs and may simplify the surgical technique.
The main advantages of navigation are limitation of hypocorrection, which can lead to early wear and implant loosening, as well as tibial plateau subsidence. It also does help to avoid hypercorrection, which is very detrimental when using mobile-bearing UKA. Indeed, in order to avoid meniscal bearing surface dislocation in medial hyperlaxity, a bigger polyethylene is used which can then generate hypercorrection leading to early degenerative changes on the opposite knee joint compartment.
Conclusion
Unicompartmental knee navigation is reliable. The navigation of only the tibial bone cut is a reasonable option as has been shown in this study. Due to the difficulties in positioning the UKA implant, and in order to avoid hyper- or hypocorrection which are both detrimental to outcome and long-term results, the navigation of UKA is a good indication for knee navigation. Its role is invaluable in the positioning of mobile-bearing UKA, where the risk of overcorrection should not be underestimated.
Contributor Information
Dominique Saragaglia, Phone: +33-476765833, FAX: +33-476765818, Email: DSaragaglia@chu-grenoble.fr.
Frédéric Picard, Phone: +44-141-9515567, Email: frederic.picard@gjnh.scot.nhs.uk.
Ramsay Refaie, Email: ramsay2000@gmail.com.
References
- 1.Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16:9–18. doi: 10.5435/00124635-200801000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Marmor L. The Marmor knee replacement. Orthop Clin North Am. 1982;13:55–64. [PubMed] [Google Scholar]
- 3.Cartier P, Cheaib S. Unicondylar knee arthroplasty. 2-10 years of follow-up evaluation. J Arthroplasty. 1987;2:157–162. doi: 10.1016/S0883-5403(87)80023-2. [DOI] [PubMed] [Google Scholar]
- 4.Hernigou P, Deschamps G. Prothèses unicompartimentales du genou. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(Suppl 1):23–60. [PubMed] [Google Scholar]
- 5.Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;423:161–165. doi: 10.1097/01.blo.0000128285.90459.12. [DOI] [PubMed] [Google Scholar]
- 6.Scott RD. Three decades of experience with unicompartmental arthroplasty: mistakes made and lessons learned. Orthopedics. 2006;29:829–831. doi: 10.3928/01477447-20060901-36. [DOI] [PubMed] [Google Scholar]
- 7.Mercier N, Wimsey S, Saragaglia D. Long-term clinical results of the Oxford medial unicompartmental knee arthroplasty. Int Orthop. 2010;34:1137–1143. doi: 10.1007/s00264-009-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neyret P, Chatain F, Deschamps G. Matériel et options dans les prothèses unicompartimentales du genou. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(Suppl 1):48–52. [Google Scholar]
- 9.Jenny JY, Boeri C. Implantation d’une prothèse totale de genou assistée par ordinateur. Etude comparative cas-témoin avec une instrumentation traditionnelle. Rev Chir Orthop Reparatrice Appar Mot. 2001;87:645–652. [PubMed] [Google Scholar]
- 10.Saragaglia D, Picard F, Chaussard C, Montbarbon E, Leitner F, Cinquin P. Mise en place des prothèses totale du genou assistée par ordinateur: comparaison avec la technique conventionnelle. A propos d’une étude prospective randomisée de 50 cas. Rev Chir Orthop Reparatrice Appar Mot. 2001;87:18–28. [PubMed] [Google Scholar]
- 11.Saragaglia D, Chaussard C, Rubens-Duval B. Navigation as a predictor of soft tissue release during 90 cases of computer-assisted total knee arthroplasty. Orthopedics. 2006;29(10 Suppl):S137–S138. [PubMed] [Google Scholar]
- 12.Ayach A, Plaweski S, Saragaglia D (2009) Computer-assisted uni knee arthroplasty for genu varum deformity. Results of axial correction in a case-control study of 40 cases. In 9th annual meeting of CAOS-International proceedings. Wingspan, Livermore, pp 4–7
- 13.Cossey AJ, Spriggins J. The use of computer-assisted surgical navigation to prevent malalignment in unicompartmental knee arthroplasty. J Arthroplasty. 2005;20:29–34. doi: 10.1016/j.arth.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Jenny JY, Boeri C. Unicompartmental knee prosthesis implantation with a non-image-based navigation system: rationale, technique, case-control comparative study with a conventional instrumented implantation. Knee Surg Sports Traumatol Arthrosc. 2003;11:40–45. doi: 10.1007/s00167-002-0333-8. [DOI] [PubMed] [Google Scholar]
- 15.Jenny JY. Navigated unicompartmental knee replacement. Sports Med Arthrosc. 2008;16:103–107. doi: 10.1097/JSA.0b013e318172b598. [DOI] [PubMed] [Google Scholar]
- 16.Jung KA, Kim SJ, Lee SC, Hwang SH, Ahn NK. Accuracy of implantation during computer-assisted minimally invasive Oxford unicompartmental knee arthroplasty: a comparison with a conventional instrumented technique. Knee. 2010;17:387–391. doi: 10.1016/j.knee.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Perlick L, Bäthis H, Tingart M, Perlick C, Lüring C, Grifka J. Minimally invasive unicompartmental knee replacement with a nonimage-based navigation system. Int Orthop. 2004;28:193–197. doi: 10.1007/s00264-004-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberger RE, Fink C, Quirbach S, Attal R, Tecklenburg K, Hoser C. The immediate effect of navigation on implant accuracy in primary mini-invasive unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2008;16:1133–1140. doi: 10.1007/s00167-008-0618-7. [DOI] [PubMed] [Google Scholar]
- 19.Jenny JY, Boeri C. Accuracy of implantation of a unicompartmental knee arthroplasty with 2 different instrumentations: a case-controlled comparative study. J Arthroplasty. 2002;17:1016–1020. doi: 10.1054/arth.2002.34524. [DOI] [PubMed] [Google Scholar]
- 20.Larsson SE, Larsson S, Lundkvist S. Unicompartmental knee arthroplasty. A prospective consecutive series followed for six to 11 years. Clin Orthop Relat Res. 1988;232:174–181. [PubMed] [Google Scholar]
- 21.Ridgeway SR, McAuley JP, Ammeen DJ, Engh GA. The effect of alignment of the knee on the outcome of unicompartmental knee replacement. J Bone Joint Surg Br. 2002;84:351–355. doi: 10.1302/0301-620X.84B3.12046. [DOI] [PubMed] [Google Scholar]
- 22.Seon JK, Song EK, Park SJ, Yoon TR, Lee KB, Jung ST. Comparison of minimally invasive unicompartmental knee arthroplasty with or without a navigation system. J Arthroplasty. 2009;24:351–357. doi: 10.1016/j.arth.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Lim MH, Tallay A, Bartlett J. Comparative study of the use of computer assisted navigation system for axial correction in medial unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2009;17:341–346. doi: 10.1007/s00167-008-0655-2. [DOI] [PubMed] [Google Scholar]