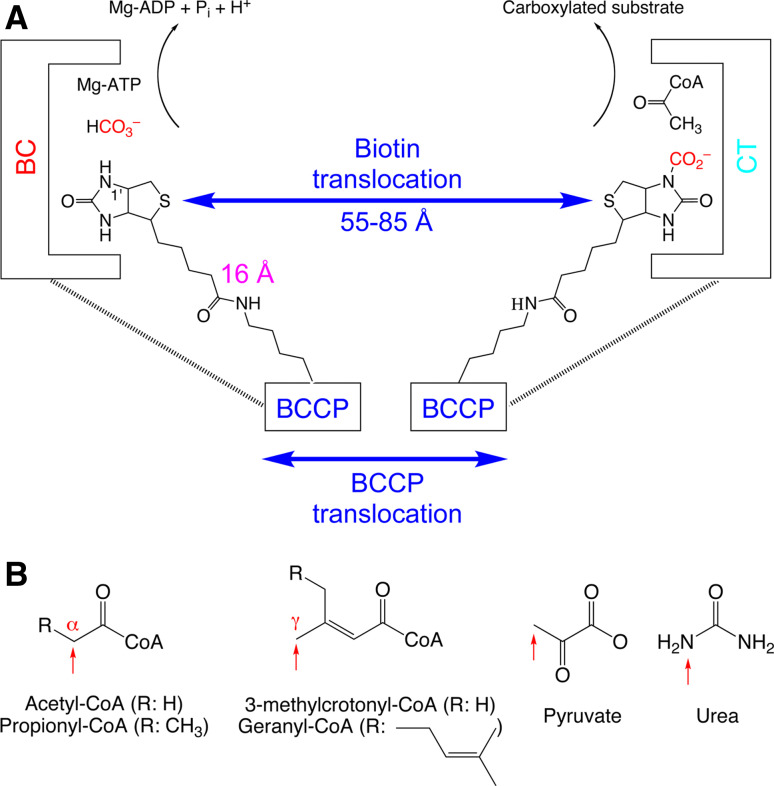
Fig. 1.
The biochemical activity of biotin-dependent carboxylases. a Biotin is carboxylated in the active site of the biotin carboxylase (BC) component, using bicarbonate as the CO2 donor with concomitant ATP hydrolysis. Biotin then translocates to the carboxyltransferase (CT) active site, where the CO2 is transferred to the acceptor (substrate, acetyl-CoA is shown as an example). In the swinging-arm model, biotin itself translocates between the BC and CT active sites, while the biotin-carboxyl carrier protein (BCCP) component remains stationary. The longest distance between the N1′ of biotin and the Cα atom of the covalently linked lysine residue is ~16 Å, giving the swinging arm a maximal reach of ~30 Å. This is significantly shorter than the distances observed in the holoenzymes so far, between 55 and 80 Å. Therefore, the BCCP domain must also translocate during catalysis, and this is known as the swinging-domain model. b The substrates of biotin-dependent carboxylases. The sites of carboxylation are indicated with the red arrows